|
|
Post by 1dave on Nov 25, 2016 8:22:28 GMT -5
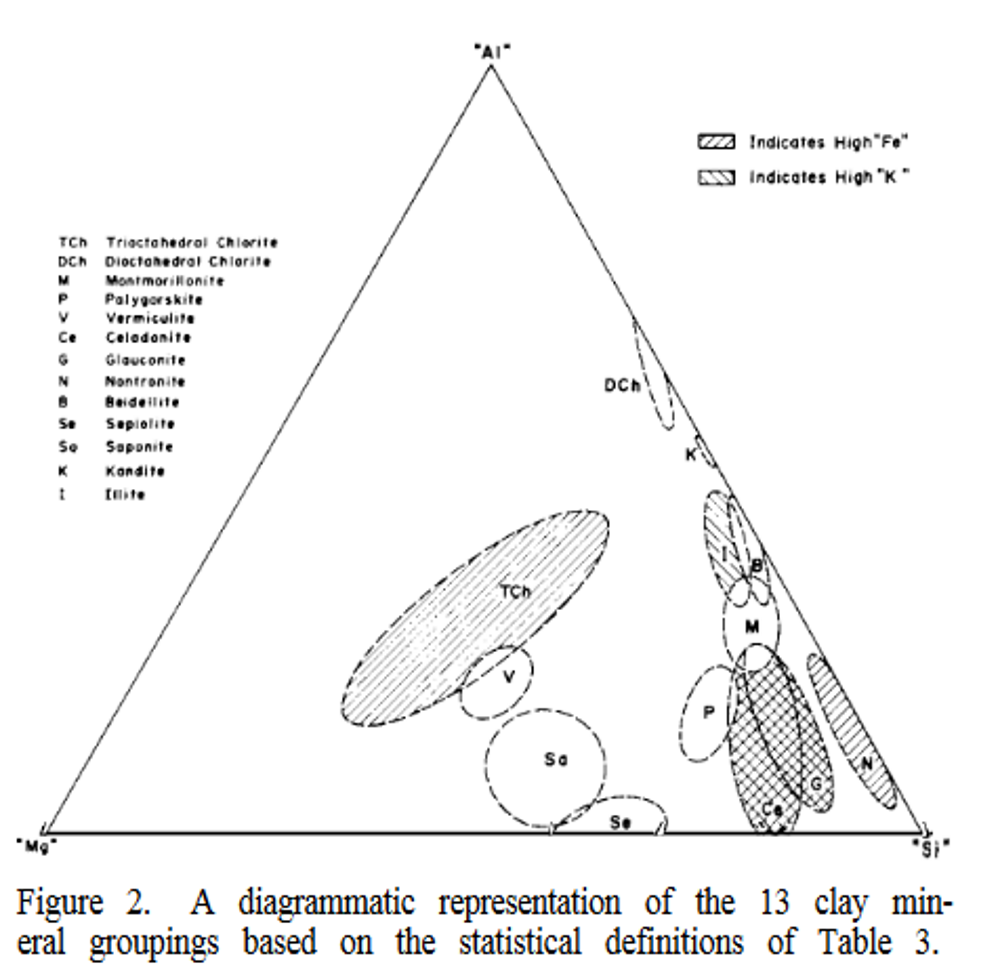
jamesp is having success using red clay as a slurry. It got me wondering so I posted: A quick dive in mud over my head. our normally visible planet Earth surface domain consists predominately of only eight elements:1 - oxygen, 2 - silicon, 3 - aluminum,4 - iron; O, s, a, i. That is easy to remember. Then 5 - calcium, 6 - sodium, 7 - potassium, 8 - magnesium; C, s, p, m. Those are the eight major rock forming elements.That is what most rocks we see are made of. Remember Sail Boat. ‘ Oh! Suddenly An Idea Came Sailing Past Me!’  O s a i; c s p m. That is what makes up ninety nine percent of mud.” That last one percent consists mostly of just eight more elements. In decreasing order, they are titanium, hydrogen, phosphorus, manganese, fluorine, barium, carbon, and strontium; T, h, p, m, f, b, c, s. The eight minor rock forming elements.‘ The Heavier Parts Made From Bigger Constructed Stars.’ That gets you to 99.9 percent of all mud.All we have to do is toss out all that is not in your RED CLAY. Clay comes from the breakdown of feldspar so we can toss out a lot of quartz - SiO2, what more? We have to keep the iron for the red, most likely aluminum because it is everywhere. Calcium,sodium, potassium, magnesium. Some of them have to be there. What do those electron shells do?
|
|
|
|
Post by 1dave on Nov 25, 2016 8:26:17 GMT -5
|
|
|
|
Post by 1dave on Nov 25, 2016 8:26:34 GMT -5
|
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,606
|
Post by jamesp on Nov 25, 2016 8:54:09 GMT -5
Well our red clay must be eat up with magnesium.
Start out with 4 pounds of boots and end up with 15 pounds of boots assuming 3 inches of sticky clay stuck to the bottom of them.
Off roaders don't need high revving engines in these parts except for one reason.
They need to rev engine up to spin tires fast enough to sling the mud off the tires to expose the tire tread to get a bite.
My shovels all have suction vents sawed into the blade so that the suction will release when raising the shovel.
Unless you like to use 50 extra pounds of lift to raise 7-8 pounds of clay in the shovel.
Our plow discs wear on the outside edge making them smaller in diameter. Effect of clay.
Sand causes plow discs to get thin and fail from lack of structural strength, rarely decreases diameter.
DOT loves our pasty red clay, when packed to the point the colloids are laying flat it becomes one of the finest road underlayment materials.
Packed red clay makes one of the finest dams in the world. Once packed and colloids are laid flat such a dam will NOT leak.
The gardening community around Atlanta despises their red clay. It takes a 5 foot wrecking bar to break into it.
Dries like concrete and stays wet so long it rots plant roots. Soil amendment business is big biz around here.
Mass composting facilities abound with giant grinders that chop oak tree stumps like confetti.
And all those dang country songs of ours about muddy rivers is due to colloidal clay that does not want to settle to the bottom of the river.
Our Mommy's whip our butts as kids because red clay does not wash out of our clothes.
We buy pick up trucks that hide the color of red clay, avoid white and black.
Gotta love red clay.
I want to know what the conductive and ionic properties of it is. No articles found thus far. Have looked and looked.
|
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,606
|
Post by jamesp on Nov 25, 2016 10:41:35 GMT -5
I knew clays were totally complicated 1dave. As they are usually a mix of other complicated clays. Not easy to find BASIC info on their conductivity or their individual effectiveness as slurry either. |
|
|
|
Post by pauls on Nov 25, 2016 15:23:10 GMT -5
Wow Dave so much information, fantastic.
I always knew clay was complex but its not easy to find information thats accesible to amateurs, thanks for a great post.
|
|
|
|
Post by MrMike on Nov 25, 2016 15:45:41 GMT -5
|
|
Deleted
Deleted Member
Member since January 1970
Posts: 0
|
Post by Deleted on Nov 25, 2016 18:15:20 GMT -5
I knew clays were totally complicated 1dave. As they are usually a mix of other complicated clays. Not easy to find BASIC info on their conductivity or their individual effectiveness as slurry either. I did some googling too. Looks like low pH will make our slurry last longer too. Unless I read it wrong, then higher pH will last longer. The flocculate differently... High pH flocs into books, and low pH flocs into house of cards shaped structure... |
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,606
|
Post by jamesp on Nov 25, 2016 22:48:13 GMT -5
I knew clays were totally complicated 1dave. As they are usually a mix of other complicated clays. Not easy to find BASIC info on their conductivity or their individual effectiveness as slurry either. I did some googling too. Looks like low pH will make our slurry last longer too. Unless I read it wrong, then higher pH will last longer. The flocculate differently... High pH flocs into books, and low pH flocs into house of cards shaped structure... I am on the low ph side. Never measured it but my skin says it is low. Water is 6.2 -6.7, mostly 6.7. Clay rich water is 6.7. Then the effects of the rocks. |
|
|
|
Post by 1dave on Nov 26, 2016 8:01:24 GMT -5
A little about jamesp 's Red Clay: So James' Red Clay weathered out later and sits on top of the Kaolin. Now, How good is it in tumbles? |
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,606
|
Post by jamesp on Nov 26, 2016 8:23:02 GMT -5
Yes 1dave. The red clay I get is above the fall line in Atlanta. The red clay hills ! Exactly. Carnegie, Rockefeller, Dupont made fortunes off of oil, real estate, railroads, chemical products, etc. Little known J.M. Huber made his fortune digging clay and probably had a financial empire rivaling the above. Almost every piece of coated paper, paint, cosmetic uses lots of kaolin. His mines can be seen from the most distant satellite photos, large operations. Red clay over burden, white kaolin below. Big operation as far as eye can see    Using a berm to keep the white kaolin from getting stained by the red clay. Neutral white color desirable for paint and paper coatings.   |
|
|
|
Post by 1dave on Nov 26, 2016 8:55:55 GMT -5
So now YOU have found a use for the previously useless!
|
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,606
|
Post by jamesp on Nov 26, 2016 9:18:06 GMT -5
So now YOU have found a use for the previously useless! Between you and the wall I took the above photos whilst stealing pure white kaolin to try in the tumbler. And it did not hold a candle to pasty red clay. That is when I surmised there must be some other property other than just colloids at work. I wet the stolen kaolin and spent time breaking it down with a maul. Then mixing it with water with a motor driven grout mixer. Then let it dry into a big cake for breaking into dose size chunks for the tumbler.     |
|
|
|
Post by 1dave on Nov 26, 2016 9:50:48 GMT -5
The new knowledge is that the slurry is as important as the abrasive.
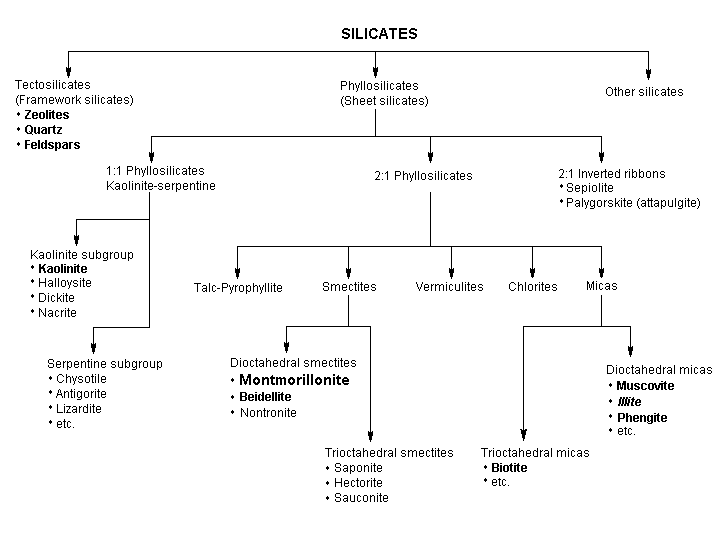
So the kaolin ( Al2Si2O5(OH)4. ) lacks the iron and magnesium. Is the iron necessary?
The black gumbo from Kansas sticks to your shoes - up to SIX INCHES!
|
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,606
|
Post by jamesp on Nov 26, 2016 10:08:48 GMT -5
The new knowledge is that the slurry is as important as the abrasive. So the kaolin ( Al2Si2O5(OH)4. ) lacks the iron and magnesium. is the iron necessary? The black gumbo from Kansas sticks to your shoes - up to SIX INCHES! I will guarantee that electrical conductivity and ion type attraction forces have all-to-do with clay slurries. I have no knowledge on the subject of conductivity. But suspensions in ALL types of slurries be it for drilling or carrying ore particles or whatever have performance related to high conductivities.(reading articles) Perhaps iron is the reason for a high conductivity ? Reduced silica and high clay content raises conductivity, that's a fact. Kaolin has another benefit when tumbling. It is like 30% aluminum oxide. And after the coarse SiC breaks down I believe the AO in the kaolin slurry is laying down a fine pre-pre-polish finish. Take the rocks out of some well broken down SiC 30 running in the rotary and put them in the vibe with AO 500 and they have a darn nice polish the next day. Just a by product. Main goal is to have a slurry that carries bigger SiC particles with high attraction(suction) forces to grind rocks faster. Coarse grind sloooow |
|
|
|
Post by 1dave on Nov 26, 2016 10:25:44 GMT -5
Sodium increases conductivity in water.
|
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,606
|
Post by jamesp on Nov 26, 2016 10:37:17 GMT -5
Sodium increases conductivity in water. Could it be ? We are not normally salty up this way. I suppose clay being agitated when wet may have a lot of things going on in it. Conductivity causing molecular attraction ? No clue Dave. |
|
Deleted
Deleted Member
Member since January 1970
Posts: 0
|
Post by Deleted on Nov 26, 2016 11:46:22 GMT -5
Sodium increases conductivity in water. Could it be ? We are not normally salty up this way. I suppose clay being agitated when wet may have a lot of things going on in it. Conductivity causing molecular attraction ? No clue Dave. The charges in the clay particles are more like a static charge. Experimentation is needed, but I suspect adding salt to the slurry will actually kill it's ability to do its job. |
|
Deleted
Deleted Member
Member since January 1970
Posts: 0
|
Post by Deleted on Nov 26, 2016 11:49:21 GMT -5
The new knowledge is that the slurry is as important as the abrasive.So the kaolin ( Al2Si2O5(OH)4. ) lacks the iron and magnesium. Is the iron necessary? The black gumbo from Kansas sticks to your shoes - up to SIX INCHES! There are grades of kaolin clay. Not really 'pure' vs not. The white stuff likely doesn't stick to boots like gumbo, or Jims red, or even the stuff a Salton sea sticking 6+" as well. I bet all of those sticky ones will work. Iron or not. The sticky nature is due to shapes of particles... |
|
|
|
Post by 1dave on Nov 26, 2016 12:15:55 GMT -5
Just stating a fact on salt. ALL metals are conductive. Conductivity is just the opposite of resistance. So instead of all the expensive equipment you can just use a simple meter from Walmart or Home Depot.  More. More. |
|