Post by 1dave on Feb 14, 2015 14:15:42 GMT -5
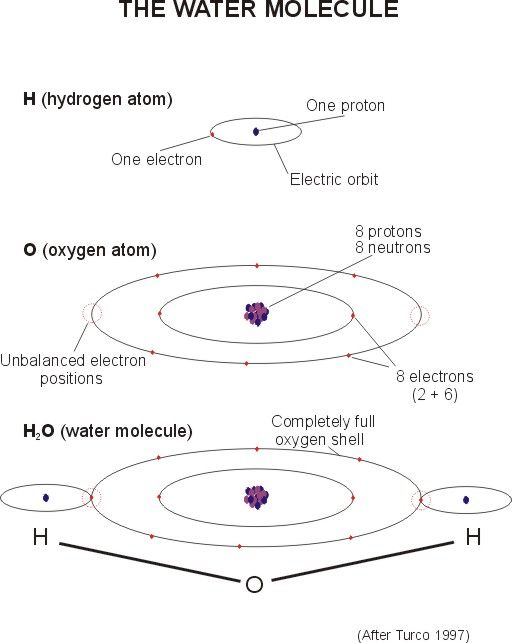
Water: Two hydrogen atoms, one oxygen atom. Simple, right?
Construction of the water molecule from H and O.JPG
The two hydrogen atoms are powerfully attached to the oxygen atoms, but don't align themselves straight across from each other at 180 degrees.
1. Oh no! Instead, they choose to locate at about 104.5 degrees from each other, making the molecule a dipole, positive on the hydrogen side and negative on the oxygen side.
Why? What force keeps them at that angle?
Jefferson Labs - Questions and answers
Newton - Ask a scientist
2,200 kilometers per second in a space usually smaller than 2 angstroms - That is so fast it is virtually everywhere at the same time! So what keeps all those quadrillions of water molecules locked in an angle of 105 degrees?
SiO2 atoms maintain an angle of 109 degrees.
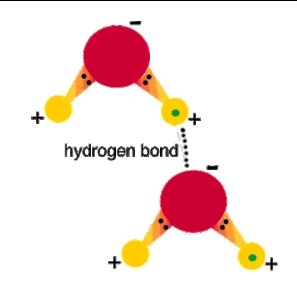
2. That allows water molecules to weakly attach to other water molecules in a variety of interesting liquid configurations - chains, ribbons, sheets, spheres, . . .
3. That polarity is also why water is called the universal solvent because more substances dissolve in water than in any other chemical. The slight positive hydrogen charge and slight negative oxygen charge helps water dissociate ionic compounds into their positive and negative ions. The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of water, dissolving them.
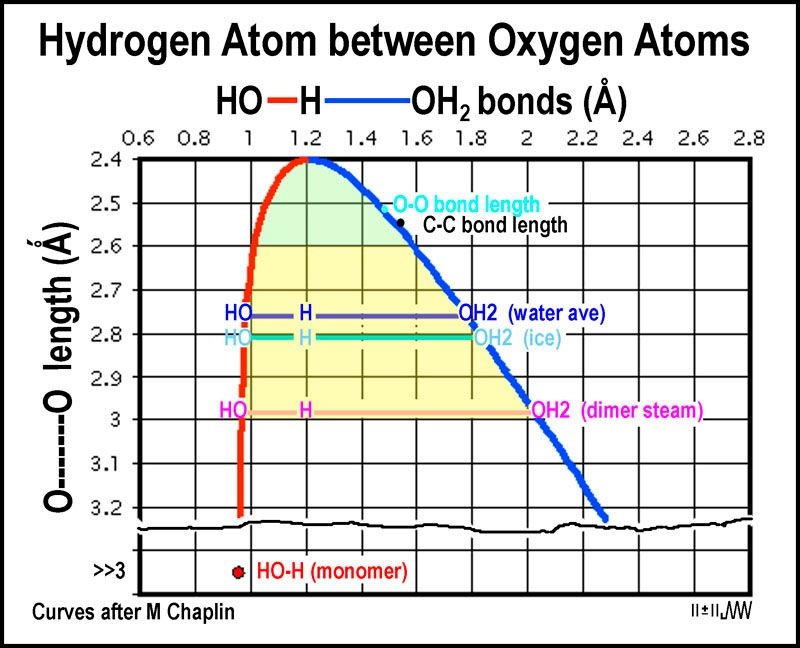
5. As temperature changes, the lengths of those "hydrogen bonds" change.
1--O-H-O bond lengths.jpg
6. Also the electrons change orbits.
This allows some interesting things to happen.
1DenSpHeat-07.jpg
Why the different angles of symmetry? They sure effect our world!
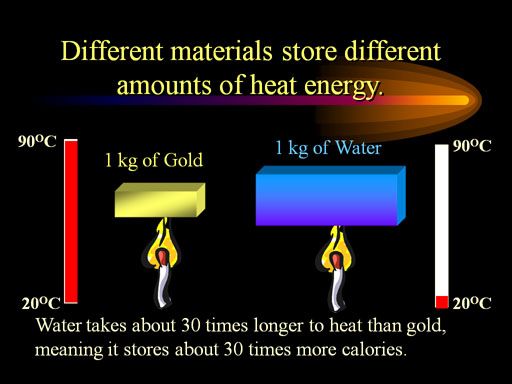
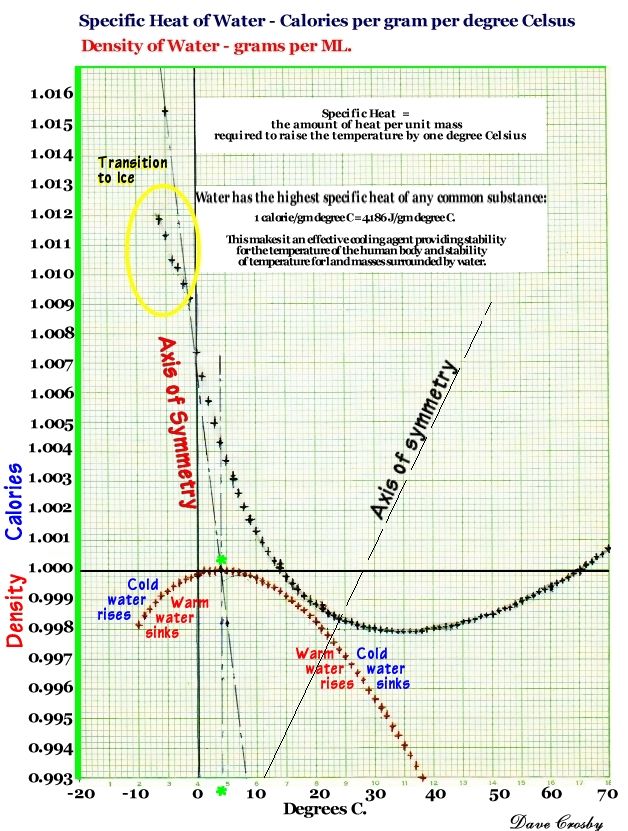
7. Some molecules pick up extra heat and become vapors. This happens most often with hot water, which has a low specific gravity. as water cools, it becomes heavier (the red curve above), but also it's ability to hold heat decreases - then increases!(the black curve). These two facts have enormous consequences on our world and in our lives.
Water has the highest specific heat capacity of any common substance!
Pure Water =1.00 in Calories per gram in degrees C.
Wet mud = 0.60, Ice (0°C) = 0.50, Steam = 0.48, Wood = 0.41, Sandy clay = 0.33, dry Air at sea level = 0.24, Asphalt = 0.22, Aluminum = 0.22, Quartz sand = 0.19, Granite = 0.19, Bone = 0.11, Copper = 0.093, Silver = 0.056, Mercury = 0.033, Lead = 0.031, Gold = 0.031.
8. When water boils above 100 degrees C, it tries to expand up to 2,000 times its original volume.
That terrifying force can rupture steel containers and blow the tops off volcanoes.
When water freezes the angle changes to Around 109 degrees, and even more strange things happen.
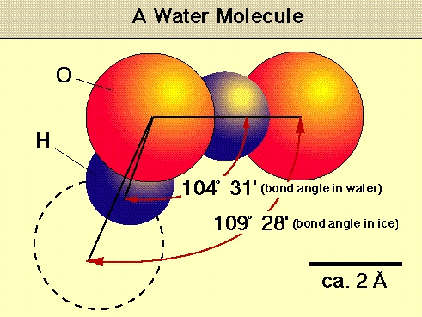
watermolecule1.gif
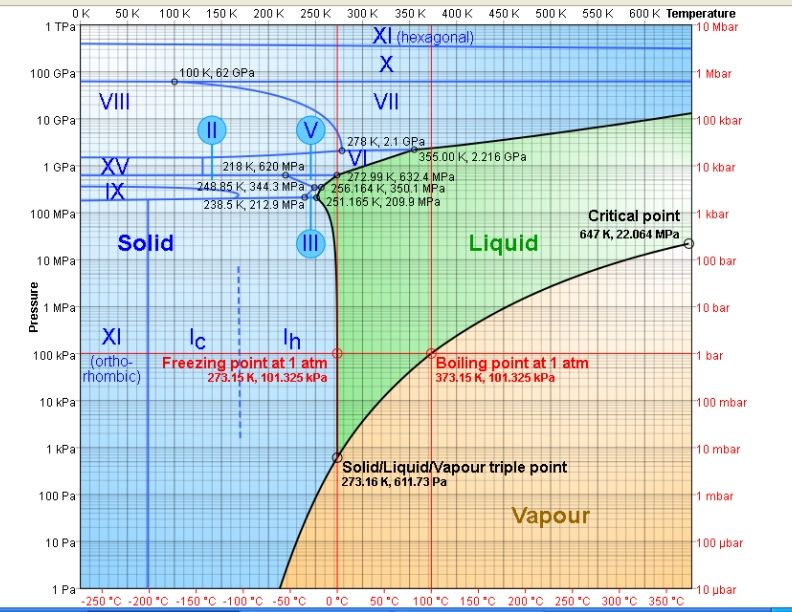
9. Ice is one of the few substances that gets lighter as it freezes.
Experimenters have verified it can form into at least 16 different crystal configurations.
1-H2O-PhaseDiagram.JPG
Even just the first few configurations can get complicated.
www1.lsbu.ac.uk/water/ice_phases.html
The Ice Rules:
The 'ice rules': (also called the Bernal–Fowler rules]) each water molecule (right labeled 'a') has four hydrogen-bonded neighbors, two hydrogen atoms near each oxygen (~1 Å), one hydrogen atom on each O····O bond; thus H-O-H···OH2 and H2O···H-O-H are allowed but H-O-H···H-O-H and H2O···OH2 are not; see H2O molecule a below).
As the H-O-H angles are about 106.6º, the hydrogen bonds are not straight (although shown so in the figures).
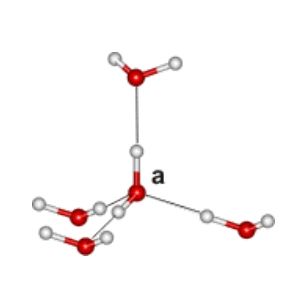
Ice Rules
Weaknesses (Bjerrum defects) in the ice crystal are apparent where the ice rules are disobeyed. Both O····O contacts, without an intervening proton (L defect, 'leer' defect) and O-H····H-O contacts (D defect, 'doppelt' defect, with two protons between the pair of oxygen atoms) may occur due to molecular rotations where neighboring water molecules fail to adjust their hydrogen bonding.
Another type of defect is the ionic defect caused by the presence of H3O+ and OH- ions.
10. I find it fascinating the way the structures change.
Ice Ih Normal hexagonal crystalline ice, has a density less than liquid water, of 0.917 g/cm³, due to the extremely low density of its crystal lattice. The density of ice Ih increases with decreasing temperature (density of ice at -180 °C is 0.9340 g/cm³). Virtually all ice in the biosphere is ice Ih, with the exception only of a small amount of ice Ic.
Ice Ic (pronounced "ice one c" or "ice icy") is a metastable cubic crystalline variant of ice. The oxygen atoms are arranged in a diamond structure. It is produced at temperatures between 130 and 220 K (-140 and -50 °C), and can exist up to 240 K,[1][2] when it transforms into ice Ih. It may occasionally be present in the upper atmosphere.[3]
Ice II is a rhombohedral crystalline form of ice with highly ordered structure. It is formed from ice Ih by compressing it at temperature of 198 K at 300 MPa or by decompressing ice V. When heated it undergoes transformation to ice III.[1] Ordinary water ice is known as ice Ih, (in the Bridgman nomenclature). Different types of ice, from ice II to ice XVI, have been created in the laboratory at different temperatures and pressures. It is thought that the cores of icy moons like Jupiter's Ganymede may be made of ice II.

1-GuideToSnowTypes4.jpg
Amorphous ice is an ice lacking crystal structure. Amorphous ice exists in three forms: low-density (LDA) formed at atmospheric pressure, or below, high density (HDA) and very high density amorphous ice (VHDA), forming at higher pressures. LDA forms by extremely quick cooling of liquid water ("hyperquenched glassy water", HGW), by depositing water vapour on very cold substrates ("amorphous solid water", ASW) or by heating high density forms of ice at ambient pressure ("LDA").
Having produced ice III, Tammann then tried condensing the ice at a temperature between -70 and -80 degrees Celsius under 2 kilobars of pressure. Tammann noted that in this state ice II was denser than he had observed ice III to be. He also found that both types of ice can be kept at normal atmospheric pressure in a stable condition so long as the temperature is kept at that of liquid air, which slows the change in conformation back to ice Ih.[2]
In later experiments by Bridgman in 1912, it was shown that the difference in volume between ice II and ice III was in the range of 0.0001 meters cubed per kilogram. This difference hadn't been discovered by Tammann due to the small change and was why he had been unable to determine an equilibrium curve between the two.
Ice III is a tetragonal form of solid matter which consists of tetragonal crystalline ice, formed by cooling water down to 250 K at 300 MPa. It is the least dense of the high-pressure water phases. Denser than water, with a density of 1160 kg/m3 (at 350 MPa). The proton-ordered form of ice III is ice IX.
Ice IV A metastable rhombohedral phase. It can be formed by heating high-density amorphous ice slowly at a pressure of 810 MPa. It doesn't form easily without a nucleating agent.[52]
Ice V A monoclinic crystalline phase. Formed by cooling water to 253 K at 500 MPa. Most complicated structure of all the phases.[53]
Ice VI A tetragonal crystalline phase. Formed by cooling water to 270 K at 1.1 GPa. Exhibits Debye relaxation.[54] two interpenetrating frameworks.
Ice VII A cubic phase. The hydrogen atoms' positions are disordered. Exhibits Debye relaxation. The hydrogen bonds form two interpenetrating ice Ic frameworks. It has a density of about 1.65 g cm-3 (at 2.5 GPa and 25 °C),[8] which is less than twice the cubic ice density as the intra-network O····O distances are 8% longer (at 0.1 MPa) to allow for interpenetration. The cubic unit cell has a side length of 3.3501 Å (for D2O, at 2.6 GPa and 22 °C) and contains two water molecules.[6]
Ice VIII A more ordered tetragonal version of ice VII, where the hydrogen atoms assume fixed positions. It is formed from ice VII, by cooling it below 5 °C (278 K).
Ice IX A tetragonal phase. Formed gradually from ice III by cooling it from 208 K to 165 K, stable below 140 K and pressures between 200 MPa and 400 MPa. It has density of 1.16 g/cm3, slightly higher than ordinary ice.
Ice X Proton-ordered cubic symmetric ice. Forms at about 70 GPa.[55]
Ice XI An orthorhombic, low-temperature equilibrium form of hexagonal ice. It is ferroelectric. Ice XI is considered the most stable configuration of ice Ih. The natural transformation process is very slow and ice XI has been found in Antarctic ice 100 to 10,000 years old. That study indicated that the temperature below which ice XI forms is -36 °C (240 K).[56]
Ice XII A tetragonal, metastable, dense crystalline phase. It is observed in the phase space of ice V and ice VI. It can be prepared by heating high-density amorphous ice from 77 K to about 183 K at 810 MPa. It has a density of 1.3 g cm-3 at 127 K (i.e., approximately 1.3 times more dense than water).
Ice XIII A monoclinic crystalline phase. Formed by cooling water to below 130 K at 500 MPa. The proton-ordered form of ice V.[57]
Ice XIV An orthorhombic crystalline phase. Formed below 118 K at 1.2 GPa. The proton-ordered form of ice XII.[57]
Ice XV The pseudo-orthorhombic proton-ordered form of ice VI formed by cooling water to around 80–108 K at 1.1 GPa.
Ice XVI The least dense crystalline form of water, cubic, topologically equivalent to the empty structure of sII Clathrate hydrates.
I expected Ice XVI to be the most compact and heaviest, but . . .

Construction of the water molecule from H and O.JPG
The two hydrogen atoms are powerfully attached to the oxygen atoms, but don't align themselves straight across from each other at 180 degrees.
1. Oh no! Instead, they choose to locate at about 104.5 degrees from each other, making the molecule a dipole, positive on the hydrogen side and negative on the oxygen side.
Why? What force keeps them at that angle?
Jefferson Labs - Questions and answers
You may wonder how fast the electrons are whizzing around in the atoms around you. A good example (and the most simple to calculate) is the hydrogen atom which is in all our water. A calculation shows that the electron is traveling at about 2,200 kilometers per second. That's less than 1% of the speed of light, but it's fast enough to get it around the Earth in just over 18 seconds.
Newton - Ask a scientist
Question: Exactly how fast do electrons travel?
Replies: Electrons can have a wide range of speeds. A slow case: we know that electrons move when there is a current flow in a wire, but the speed at which the electrons themselves move in the wire -- the so-called electron drift velocity -- surprises most people. For example, for a copper wire of radius 1 mm carrying a steady current of 10 amps, the drift velocity is only about 0.024 cm/sec ! On the fast side: the Bohr model of the hydrogen atom has the (bound) electron zipping around the nucleus at about 2 million meters/sec. And on the very fast side, some examples are: beta particles, which are emitted by some radioactive materials; and the innermost electrons of atoms of elements having large atomic number, such as uranium. In these cases the electrons are traveling at very nearly the speed of light. (about 300 million meters/sec).
Rcwinther
Replies: Electrons can have a wide range of speeds. A slow case: we know that electrons move when there is a current flow in a wire, but the speed at which the electrons themselves move in the wire -- the so-called electron drift velocity -- surprises most people. For example, for a copper wire of radius 1 mm carrying a steady current of 10 amps, the drift velocity is only about 0.024 cm/sec ! On the fast side: the Bohr model of the hydrogen atom has the (bound) electron zipping around the nucleus at about 2 million meters/sec. And on the very fast side, some examples are: beta particles, which are emitted by some radioactive materials; and the innermost electrons of atoms of elements having large atomic number, such as uranium. In these cases the electrons are traveling at very nearly the speed of light. (about 300 million meters/sec).
Rcwinther
2,200 kilometers per second in a space usually smaller than 2 angstroms - That is so fast it is virtually everywhere at the same time! So what keeps all those quadrillions of water molecules locked in an angle of 105 degrees?
Electromagnetic forces!
SiO2 atoms maintain an angle of 109 degrees.

2. That allows water molecules to weakly attach to other water molecules in a variety of interesting liquid configurations - chains, ribbons, sheets, spheres, . . .
3. That polarity is also why water is called the universal solvent because more substances dissolve in water than in any other chemical. The slight positive hydrogen charge and slight negative oxygen charge helps water dissociate ionic compounds into their positive and negative ions. The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of water, dissolving them.
5. As temperature changes, the lengths of those "hydrogen bonds" change.

1--O-H-O bond lengths.jpg
6. Also the electrons change orbits.

This allows some interesting things to happen.

1DenSpHeat-07.jpg
Why the different angles of symmetry? They sure effect our world!
7. Some molecules pick up extra heat and become vapors. This happens most often with hot water, which has a low specific gravity. as water cools, it becomes heavier (the red curve above), but also it's ability to hold heat decreases - then increases!(the black curve). These two facts have enormous consequences on our world and in our lives.
Water has the highest specific heat capacity of any common substance!
Pure Water =1.00 in Calories per gram in degrees C.
Wet mud = 0.60, Ice (0°C) = 0.50, Steam = 0.48, Wood = 0.41, Sandy clay = 0.33, dry Air at sea level = 0.24, Asphalt = 0.22, Aluminum = 0.22, Quartz sand = 0.19, Granite = 0.19, Bone = 0.11, Copper = 0.093, Silver = 0.056, Mercury = 0.033, Lead = 0.031, Gold = 0.031.
8. When water boils above 100 degrees C, it tries to expand up to 2,000 times its original volume.
That terrifying force can rupture steel containers and blow the tops off volcanoes.
When water freezes the angle changes to Around 109 degrees, and even more strange things happen.
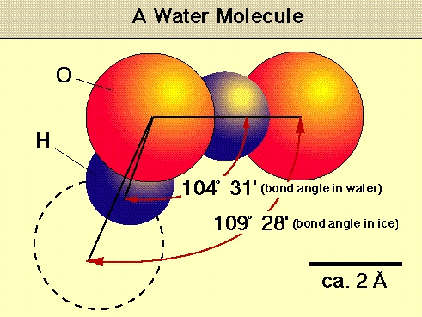
watermolecule1.gif
9. Ice is one of the few substances that gets lighter as it freezes.
Experimenters have verified it can form into at least 16 different crystal configurations.

1-H2O-PhaseDiagram.JPG
Even just the first few configurations can get complicated.
www1.lsbu.ac.uk/water/ice_phases.html
The Ice Rules:
The 'ice rules': (also called the Bernal–Fowler rules]) each water molecule (right labeled 'a') has four hydrogen-bonded neighbors, two hydrogen atoms near each oxygen (~1 Å), one hydrogen atom on each O····O bond; thus H-O-H···OH2 and H2O···H-O-H are allowed but H-O-H···H-O-H and H2O···OH2 are not; see H2O molecule a below).
As the H-O-H angles are about 106.6º, the hydrogen bonds are not straight (although shown so in the figures).

Ice Rules
Weaknesses (Bjerrum defects) in the ice crystal are apparent where the ice rules are disobeyed. Both O····O contacts, without an intervening proton (L defect, 'leer' defect) and O-H····H-O contacts (D defect, 'doppelt' defect, with two protons between the pair of oxygen atoms) may occur due to molecular rotations where neighboring water molecules fail to adjust their hydrogen bonding.
Another type of defect is the ionic defect caused by the presence of H3O+ and OH- ions.
10. I find it fascinating the way the structures change.
Ice Ih Normal hexagonal crystalline ice, has a density less than liquid water, of 0.917 g/cm³, due to the extremely low density of its crystal lattice. The density of ice Ih increases with decreasing temperature (density of ice at -180 °C is 0.9340 g/cm³). Virtually all ice in the biosphere is ice Ih, with the exception only of a small amount of ice Ic.
Ice Ic (pronounced "ice one c" or "ice icy") is a metastable cubic crystalline variant of ice. The oxygen atoms are arranged in a diamond structure. It is produced at temperatures between 130 and 220 K (-140 and -50 °C), and can exist up to 240 K,[1][2] when it transforms into ice Ih. It may occasionally be present in the upper atmosphere.[3]
Ice II is a rhombohedral crystalline form of ice with highly ordered structure. It is formed from ice Ih by compressing it at temperature of 198 K at 300 MPa or by decompressing ice V. When heated it undergoes transformation to ice III.[1] Ordinary water ice is known as ice Ih, (in the Bridgman nomenclature). Different types of ice, from ice II to ice XVI, have been created in the laboratory at different temperatures and pressures. It is thought that the cores of icy moons like Jupiter's Ganymede may be made of ice II.

1-GuideToSnowTypes4.jpg
Amorphous ice is an ice lacking crystal structure. Amorphous ice exists in three forms: low-density (LDA) formed at atmospheric pressure, or below, high density (HDA) and very high density amorphous ice (VHDA), forming at higher pressures. LDA forms by extremely quick cooling of liquid water ("hyperquenched glassy water", HGW), by depositing water vapour on very cold substrates ("amorphous solid water", ASW) or by heating high density forms of ice at ambient pressure ("LDA").
Having produced ice III, Tammann then tried condensing the ice at a temperature between -70 and -80 degrees Celsius under 2 kilobars of pressure. Tammann noted that in this state ice II was denser than he had observed ice III to be. He also found that both types of ice can be kept at normal atmospheric pressure in a stable condition so long as the temperature is kept at that of liquid air, which slows the change in conformation back to ice Ih.[2]
In later experiments by Bridgman in 1912, it was shown that the difference in volume between ice II and ice III was in the range of 0.0001 meters cubed per kilogram. This difference hadn't been discovered by Tammann due to the small change and was why he had been unable to determine an equilibrium curve between the two.
Ice III is a tetragonal form of solid matter which consists of tetragonal crystalline ice, formed by cooling water down to 250 K at 300 MPa. It is the least dense of the high-pressure water phases. Denser than water, with a density of 1160 kg/m3 (at 350 MPa). The proton-ordered form of ice III is ice IX.
Ice IV A metastable rhombohedral phase. It can be formed by heating high-density amorphous ice slowly at a pressure of 810 MPa. It doesn't form easily without a nucleating agent.[52]
Ice V A monoclinic crystalline phase. Formed by cooling water to 253 K at 500 MPa. Most complicated structure of all the phases.[53]
Ice VI A tetragonal crystalline phase. Formed by cooling water to 270 K at 1.1 GPa. Exhibits Debye relaxation.[54] two interpenetrating frameworks.
Ice VII A cubic phase. The hydrogen atoms' positions are disordered. Exhibits Debye relaxation. The hydrogen bonds form two interpenetrating ice Ic frameworks. It has a density of about 1.65 g cm-3 (at 2.5 GPa and 25 °C),[8] which is less than twice the cubic ice density as the intra-network O····O distances are 8% longer (at 0.1 MPa) to allow for interpenetration. The cubic unit cell has a side length of 3.3501 Å (for D2O, at 2.6 GPa and 22 °C) and contains two water molecules.[6]
Ice VIII A more ordered tetragonal version of ice VII, where the hydrogen atoms assume fixed positions. It is formed from ice VII, by cooling it below 5 °C (278 K).
Ice IX A tetragonal phase. Formed gradually from ice III by cooling it from 208 K to 165 K, stable below 140 K and pressures between 200 MPa and 400 MPa. It has density of 1.16 g/cm3, slightly higher than ordinary ice.
Ice X Proton-ordered cubic symmetric ice. Forms at about 70 GPa.[55]
Ice XI An orthorhombic, low-temperature equilibrium form of hexagonal ice. It is ferroelectric. Ice XI is considered the most stable configuration of ice Ih. The natural transformation process is very slow and ice XI has been found in Antarctic ice 100 to 10,000 years old. That study indicated that the temperature below which ice XI forms is -36 °C (240 K).[56]
Ice XII A tetragonal, metastable, dense crystalline phase. It is observed in the phase space of ice V and ice VI. It can be prepared by heating high-density amorphous ice from 77 K to about 183 K at 810 MPa. It has a density of 1.3 g cm-3 at 127 K (i.e., approximately 1.3 times more dense than water).
Ice XIII A monoclinic crystalline phase. Formed by cooling water to below 130 K at 500 MPa. The proton-ordered form of ice V.[57]
Ice XIV An orthorhombic crystalline phase. Formed below 118 K at 1.2 GPa. The proton-ordered form of ice XII.[57]
Ice XV The pseudo-orthorhombic proton-ordered form of ice VI formed by cooling water to around 80–108 K at 1.1 GPa.
Ice XVI The least dense crystalline form of water, cubic, topologically equivalent to the empty structure of sII Clathrate hydrates.
I expected Ice XVI to be the most compact and heaviest, but . . .