Star Stones, Titanium, and The Anorthosite puzzle.
Sept 16, 2017 19:26:59 GMT -5
RWA3006, kk, and 1 more like this
Post by 1dave on Sept 16, 2017 19:26:59 GMT -5
Star Stones, Titanium, and The Anorthosite puzzle.


A. What is Titanium?
Titanium greatly enriches our rockhound world with Rutilated Quartz Crystals, in the asterism of Rose Quartz, Star Garnet, Ruby and Sapphire. More about that later.

Titanium is a chemical element with symbol Ti and atomic number 22.
Properties: Titanium has a melting point of 1660 +/- 10°C, boiling point of 3287°C, specific gravity of 4.54, with a valence of 2, 3, or 4.
It is a lustrous transition metal (an element whose atom has a partially filled d sub-shell)with a silver color, low density, and high strength. Titanium is resistant to corrosion in sea water, aqua regia, and chlorine. It is non-magnetic and a poor conductor of heat and electricity.
It is one of the few elements that burns in pure nitrogen gas - to form brittle titanium nitride.
A 2012 study of a particular dying star, Supernova 1987A, found that a single supernova can create 100 Earths worth of titanium.
It was named after the Greek Titan Gods because of it’s strength, is the ninth-most abundant element in Earth's crust (0.63% by mass).
It is present as oxides in most igneous rocks, in sediments derived from them, in living things, and natural bodies of water.
Its proportion in soils is approximately 0.5 to 1.5%.
Of the 801 types of igneous rocks analyzed by the United States Geological Survey, 784 contained titanium.
Titanium becomes radioactive upon bombardment with deuterons, emitting mainly positrons and hard gamma rays.
Naturally occurring titanium is composed of 5 stable isotopes: 46Ti, 47Ti, 48Ti, 49Ti, and 50Ti, with 48Ti being the most abundant at 73.8%. Eleven radioisotopes have been recognized, the most stable being 44Ti with a half-life of 63 years and the majority, less than half a second.
Of all the metals, Titanium is the most compatible with human flesh and bones, and is used extensively in surgery procedures.
As Titanium is heavier than most surface rocks, but lighter than iron, it tends to accumulate between the mantle and crust.
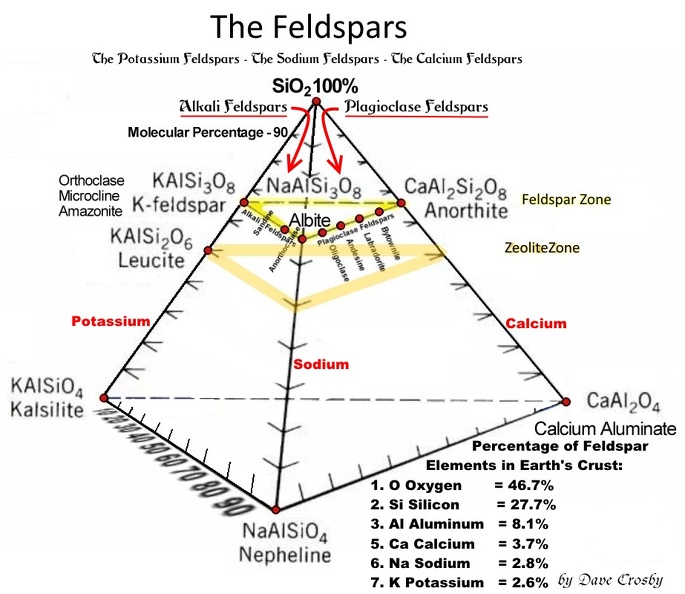
The Five Main Mineral Forms (+ 2 New) found are:

1. Ilmenite - iron titanium oxide, FeTiO3 - Trigonal Rhombohedral.
Ilmenite is commonly recognized in altered igneous rocks by the presence of a white, brown, or dark alteration product, the “pseudo-mineral leucoxene.” Often ilmenites are rimmed with leucoxene, an alteration product and mixture of Fe-Ti oxides - including titanite, perovskite, titanian magnetite, but especially ilmenite - which allows ilmenite to be distinguished from magnetite and other iron-titanium oxides. . Most 'leucoxene' is anatase or rutile.
The example shown in the image above is typical of leucoxene-rimmed ilmenite.

2. Sphene / Titanite - calcium titanium silicate, CaTiSiO5 - Monoclinic Prismatic.
Titanite occurs as a common accessory mineral in intermediate and felsic igneous rocks and associated pegmatites. It also occurs in metamorphic rocks such as gneiss and schists and skarns.
The most important oxide is TiO2, which exists in three polymorphs:

3. Rutile - Tetragonal Ditetragonal dipyramidal - its name comes from Latin rutilus, red, because of the deep red color observed in some specimens when viewed by transmitted light.
At 6-6.5 in hardness on the Mohs scale, and specific gravity about 4.2,
it is the most stable polymorph of TiO2 at all temperatures, exhibiting lower total free energy than the metastable phases of anatase or brookite. Consequently, the transformation of the metastable TiO2 polymorphs to rutile is irreversible. As it has the lowest molecular volume of the three main polymorphs; it is generally the primary titanium bearing phase in most high-pressure metamorphic rocks, chiefly eclogites.


Rutile has among the highest refractive indices at visible wavelengths of any known crystal, and also exhibits a particularly large birefringence and high dispersion making it useful in polarization optics.
Rutile is a common accessory mineral in plutonic igneous rocks, though it is also found occasionally in extrusive igneous rocks, particularly those that have deep mantle sources such as kimberlites and lamproites.
Natural rutile may contain up to 10% iron and significant amounts of niobium and tantalum.
The occurrence of large specimen crystals is most common in pegmatites, skarns, and granite greisens. Rutile is found as an accessory mineral in some altered igneous rocks, and in certain gneisses and schists.
Rutile crystals are most commonly observed to exhibit a prsimatic or acicular growth habit with preferential orientation along their c-axis, the [001] direction. This growth habit is favored as the {110} facets of rutile exhibit the lowest surface free energy and are therefore thermodynamically most stable. The c-axis oriented growth of rutile appears clearly in abnormal grain growth nanorods and nanowires phenomena of this phase.
In groups of acicular crystals it is frequently seen penetrating quartz as in the “fléches d'amour” from Graubünden, Switzerland. As quartz has a hardness of 7 and rutile is a 6, it can be difficult to cut and polish a rutilated stone without creating pits and cracks in the quartz.
Small rutile needles present in gems are responsible for an optical phenomenon known as asterism. Asteriated gems are known as "star" gems. Star sapphires, star rubies, and other "star" gems are highly sought after and are generally more valuable than their normal counterparts.
The other main polymorphs, Anatase and brookite, are found in igneous environments as products of autogenic alteration during the cooling of plutonic rocks; anatase is also found in placer deposits of primary rutile.

4. Anatase - Tetragonal Ditetragonal dipyramidal -
It is always found as small, isolated and sharply developed crystals, and like rutile, it crystallizes in the tetragonal system; but the common pyramid faces of anatase, which have perfect cleavages, has an angle of 82°9', while the corresponding angle of rutile is 56°52½'. Because of the steeper pyramid of anatase the mineral was named from the Greek anatasis, "extension", by René Just Haüy in 1801. There are also important differences between the physical characters of anatase and rutile: the former is less hard (5.5–6 vs. 6-6.5 Mohs) and dense (specific gravity about 3.9 vs. 4.2). Also, anatase is optically negative whereas rutile is positive, and its luster is even more strongly adamantine or metallic-adamantine than that of rutile.
Two growth habits of anatase crystals may be distinguished.
The more common occurs as simple acute double pyramids with an indigo-blue to black color and steely luster. Crystals of this kind are abundant at Le Bourg-d'Oisans in Dauphiné, where they are associated with rock-crystal, feldspar, and axinite in crevices in granite and mica-schist. Similar crystals, but of microscopic size, are widely distributed in sedimentary rocks, such as sandstones, clays, and slates, from which they may be separated by washing away the lighter constituents of the powdered rock.[5]
Crystals of the second type have numerous pyramidal faces developed, and they are usually flatter or sometimes prismatic in habit; the color is honey-yellow to brown. Such crystals closely resemble xenotime in appearance and, indeed, were for a long time supposed to belong to this species, the special name wiserine being applied to them. They occur attached to the walls of crevices in the gneisses of the Alps, the Binnenthal near Brig in canton Valais, Switzerland, being a well-known locality. Naturally occurring pseudomorphs of rutile after anatase are also known.
While anatase is not an equilibrium phase of TiO2, it is kinetically stabilized. At temperatures between 550 and about 1000 °C, anatase transforms to the equilibrium rutile phase, increasing its specific gravity to 4.2. The temperature of this transformation strongly depends on the impurities or dopants present in the material as well as on the morphology of the sample.

5. Brookite - Orthorhombic Dipyramidal -
Brookite is the orthorhombic variant of titanium dioxide, TiO2, which occurs in four natural polymorphic forms (minerals with the same composition but different structure). The International Mineralogical Association (IMA) recognizes these four forms; the others are akaogiite (monoclinic), anatase (tetragonal) and rutile (tetragonal). Brookite is rare compared to anatase and rutile and, like these forms, it exhibits photocatalytic activity. Brookite has a larger cell volume than either anatase or rutile, with 8 TiO2 groups per unit cell, compared with 4 for anatase and 2 for rutile. Iron Fe, tantalum Ta and niobium Nb are common impurities.
It was named in 1825 by French mineralogist Armand Lévy for Henry James Brooke (1771–1857), an English crystallographer, mineralogist and wool trader.
Arkansite is a variety of brookite from Arkansas, US, that is also found in the Murunskii Massif, in the Eastern Siberian region of Russia, where many other unusual minerals occur.
At temperatures above about 750 °C, brookite will revert to the rutile structure.
6. Akaogiite
7. TiO2 II Mindat, TiO2 II, www.mindat.org/min-29114.html
A shock-induced or ultrahigh-pressure environment, dense polymorph of TiO2 with a α-PbO2 structure.
Originally reported from Ries Crater, Nördlingen, Swabia, Bavaria, Germany. Formula:TiO2 really “The fifth polymorph of TiO2"
Colour:Colourless (synthetic)
Name:Name used until species is submitted to the IMA and approved.
Polymorph of:Akaogiite, Anatase, Brookite, Riesite, Rutile, UM1991-08-O:Ti
8. Riesite - Monoclinic Prismatic -
Type Locality: Nördlinger Ries Crater, Swabia, Bavaria, Germany
Polymorph of:Akaogiite, Anatase, Brookite, Rutile, TiO2 II, UM1991-08-O:Ti
The sixth polymorph of TiO2.
Classification of Riesite - IMA status: Approved, Pending publication
Approval Year: 2016

A. What is Titanium?
Titanium greatly enriches our rockhound world with Rutilated Quartz Crystals, in the asterism of Rose Quartz, Star Garnet, Ruby and Sapphire. More about that later.

Titanium is a chemical element with symbol Ti and atomic number 22.
Properties: Titanium has a melting point of 1660 +/- 10°C, boiling point of 3287°C, specific gravity of 4.54, with a valence of 2, 3, or 4.
It is a lustrous transition metal (an element whose atom has a partially filled d sub-shell)with a silver color, low density, and high strength. Titanium is resistant to corrosion in sea water, aqua regia, and chlorine. It is non-magnetic and a poor conductor of heat and electricity.
It is one of the few elements that burns in pure nitrogen gas - to form brittle titanium nitride.
A 2012 study of a particular dying star, Supernova 1987A, found that a single supernova can create 100 Earths worth of titanium.
It was named after the Greek Titan Gods because of it’s strength, is the ninth-most abundant element in Earth's crust (0.63% by mass).
It is present as oxides in most igneous rocks, in sediments derived from them, in living things, and natural bodies of water.
Its proportion in soils is approximately 0.5 to 1.5%.
Of the 801 types of igneous rocks analyzed by the United States Geological Survey, 784 contained titanium.
Titanium becomes radioactive upon bombardment with deuterons, emitting mainly positrons and hard gamma rays.
Naturally occurring titanium is composed of 5 stable isotopes: 46Ti, 47Ti, 48Ti, 49Ti, and 50Ti, with 48Ti being the most abundant at 73.8%. Eleven radioisotopes have been recognized, the most stable being 44Ti with a half-life of 63 years and the majority, less than half a second.
Of all the metals, Titanium is the most compatible with human flesh and bones, and is used extensively in surgery procedures.
As Titanium is heavier than most surface rocks, but lighter than iron, it tends to accumulate between the mantle and crust.
The Five Main Mineral Forms (+ 2 New) found are:

1. Ilmenite - iron titanium oxide, FeTiO3 - Trigonal Rhombohedral.
Ilmenite is commonly recognized in altered igneous rocks by the presence of a white, brown, or dark alteration product, the “pseudo-mineral leucoxene.” Often ilmenites are rimmed with leucoxene, an alteration product and mixture of Fe-Ti oxides - including titanite, perovskite, titanian magnetite, but especially ilmenite - which allows ilmenite to be distinguished from magnetite and other iron-titanium oxides. . Most 'leucoxene' is anatase or rutile.
The example shown in the image above is typical of leucoxene-rimmed ilmenite.

2. Sphene / Titanite - calcium titanium silicate, CaTiSiO5 - Monoclinic Prismatic.
Titanite occurs as a common accessory mineral in intermediate and felsic igneous rocks and associated pegmatites. It also occurs in metamorphic rocks such as gneiss and schists and skarns.
The most important oxide is TiO2, which exists in three polymorphs:

3. Rutile - Tetragonal Ditetragonal dipyramidal - its name comes from Latin rutilus, red, because of the deep red color observed in some specimens when viewed by transmitted light.
At 6-6.5 in hardness on the Mohs scale, and specific gravity about 4.2,
it is the most stable polymorph of TiO2 at all temperatures, exhibiting lower total free energy than the metastable phases of anatase or brookite. Consequently, the transformation of the metastable TiO2 polymorphs to rutile is irreversible. As it has the lowest molecular volume of the three main polymorphs; it is generally the primary titanium bearing phase in most high-pressure metamorphic rocks, chiefly eclogites.

Note IMPACTS are NOT mentioned.

Rutile has among the highest refractive indices at visible wavelengths of any known crystal, and also exhibits a particularly large birefringence and high dispersion making it useful in polarization optics.
Rutile is a common accessory mineral in plutonic igneous rocks, though it is also found occasionally in extrusive igneous rocks, particularly those that have deep mantle sources such as kimberlites and lamproites.
Natural rutile may contain up to 10% iron and significant amounts of niobium and tantalum.
The occurrence of large specimen crystals is most common in pegmatites, skarns, and granite greisens. Rutile is found as an accessory mineral in some altered igneous rocks, and in certain gneisses and schists.
Rutile crystals are most commonly observed to exhibit a prsimatic or acicular growth habit with preferential orientation along their c-axis, the [001] direction. This growth habit is favored as the {110} facets of rutile exhibit the lowest surface free energy and are therefore thermodynamically most stable. The c-axis oriented growth of rutile appears clearly in abnormal grain growth nanorods and nanowires phenomena of this phase.
In groups of acicular crystals it is frequently seen penetrating quartz as in the “fléches d'amour” from Graubünden, Switzerland. As quartz has a hardness of 7 and rutile is a 6, it can be difficult to cut and polish a rutilated stone without creating pits and cracks in the quartz.
Small rutile needles present in gems are responsible for an optical phenomenon known as asterism. Asteriated gems are known as "star" gems. Star sapphires, star rubies, and other "star" gems are highly sought after and are generally more valuable than their normal counterparts.
The other main polymorphs, Anatase and brookite, are found in igneous environments as products of autogenic alteration during the cooling of plutonic rocks; anatase is also found in placer deposits of primary rutile.

4. Anatase - Tetragonal Ditetragonal dipyramidal -
It is always found as small, isolated and sharply developed crystals, and like rutile, it crystallizes in the tetragonal system; but the common pyramid faces of anatase, which have perfect cleavages, has an angle of 82°9', while the corresponding angle of rutile is 56°52½'. Because of the steeper pyramid of anatase the mineral was named from the Greek anatasis, "extension", by René Just Haüy in 1801. There are also important differences between the physical characters of anatase and rutile: the former is less hard (5.5–6 vs. 6-6.5 Mohs) and dense (specific gravity about 3.9 vs. 4.2). Also, anatase is optically negative whereas rutile is positive, and its luster is even more strongly adamantine or metallic-adamantine than that of rutile.
Two growth habits of anatase crystals may be distinguished.
The more common occurs as simple acute double pyramids with an indigo-blue to black color and steely luster. Crystals of this kind are abundant at Le Bourg-d'Oisans in Dauphiné, where they are associated with rock-crystal, feldspar, and axinite in crevices in granite and mica-schist. Similar crystals, but of microscopic size, are widely distributed in sedimentary rocks, such as sandstones, clays, and slates, from which they may be separated by washing away the lighter constituents of the powdered rock.[5]
Crystals of the second type have numerous pyramidal faces developed, and they are usually flatter or sometimes prismatic in habit; the color is honey-yellow to brown. Such crystals closely resemble xenotime in appearance and, indeed, were for a long time supposed to belong to this species, the special name wiserine being applied to them. They occur attached to the walls of crevices in the gneisses of the Alps, the Binnenthal near Brig in canton Valais, Switzerland, being a well-known locality. Naturally occurring pseudomorphs of rutile after anatase are also known.
While anatase is not an equilibrium phase of TiO2, it is kinetically stabilized. At temperatures between 550 and about 1000 °C, anatase transforms to the equilibrium rutile phase, increasing its specific gravity to 4.2. The temperature of this transformation strongly depends on the impurities or dopants present in the material as well as on the morphology of the sample.

5. Brookite - Orthorhombic Dipyramidal -
Brookite is the orthorhombic variant of titanium dioxide, TiO2, which occurs in four natural polymorphic forms (minerals with the same composition but different structure). The International Mineralogical Association (IMA) recognizes these four forms; the others are akaogiite (monoclinic), anatase (tetragonal) and rutile (tetragonal). Brookite is rare compared to anatase and rutile and, like these forms, it exhibits photocatalytic activity. Brookite has a larger cell volume than either anatase or rutile, with 8 TiO2 groups per unit cell, compared with 4 for anatase and 2 for rutile. Iron Fe, tantalum Ta and niobium Nb are common impurities.
It was named in 1825 by French mineralogist Armand Lévy for Henry James Brooke (1771–1857), an English crystallographer, mineralogist and wool trader.
Arkansite is a variety of brookite from Arkansas, US, that is also found in the Murunskii Massif, in the Eastern Siberian region of Russia, where many other unusual minerals occur.
At temperatures above about 750 °C, brookite will revert to the rutile structure.
6. Akaogiite
From Wikipedia, the free encyclopedia
Akaogiite is an exceedingly rare mineral, one of natural forms of titanium dioxide. It is the high-pressure polymorph of TiO2, polymorphous with anatase, brookite and another high-pressure phase called "TiO2 II".
References
El Goresy, A., Dubrovinsky, L., Gillet, P., Graup, G., and Chen, M., 2010: Akaogiite: An ultra-dense polymorph of TiO2 with the baddeleyite-type structure, in shocked garnet gneiss from the Ries Crater, Germany. American Mineralogist 95(5-6), 892-895
Mindat, Akaogiite, www.mindat.org/min-35912.html
Akaogiite is an exceedingly rare mineral, one of natural forms of titanium dioxide. It is the high-pressure polymorph of TiO2, polymorphous with anatase, brookite and another high-pressure phase called "TiO2 II".
References
El Goresy, A., Dubrovinsky, L., Gillet, P., Graup, G., and Chen, M., 2010: Akaogiite: An ultra-dense polymorph of TiO2 with the baddeleyite-type structure, in shocked garnet gneiss from the Ries Crater, Germany. American Mineralogist 95(5-6), 892-895
Mindat, Akaogiite, www.mindat.org/min-35912.html
7. TiO2 II Mindat, TiO2 II, www.mindat.org/min-29114.html
A shock-induced or ultrahigh-pressure environment, dense polymorph of TiO2 with a α-PbO2 structure.
Originally reported from Ries Crater, Nördlingen, Swabia, Bavaria, Germany. Formula:TiO2 really “The fifth polymorph of TiO2"
Colour:Colourless (synthetic)
Name:Name used until species is submitted to the IMA and approved.
Polymorph of:Akaogiite, Anatase, Brookite, Riesite, Rutile, UM1991-08-O:Ti
8. Riesite - Monoclinic Prismatic -
Type Locality: Nördlinger Ries Crater, Swabia, Bavaria, Germany
Polymorph of:Akaogiite, Anatase, Brookite, Rutile, TiO2 II, UM1991-08-O:Ti
The sixth polymorph of TiO2.
Classification of Riesite - IMA status: Approved, Pending publication
Approval Year: 2016