|
|
Post by parfive on Jul 22, 2022 0:25:38 GMT -5
Big biz worldwide, Bob , probably $20 billion this year. Thumler’s polish, market share: ~0.000000%.  |
|
|
|
Post by Bob on Jul 22, 2022 11:06:28 GMT -5
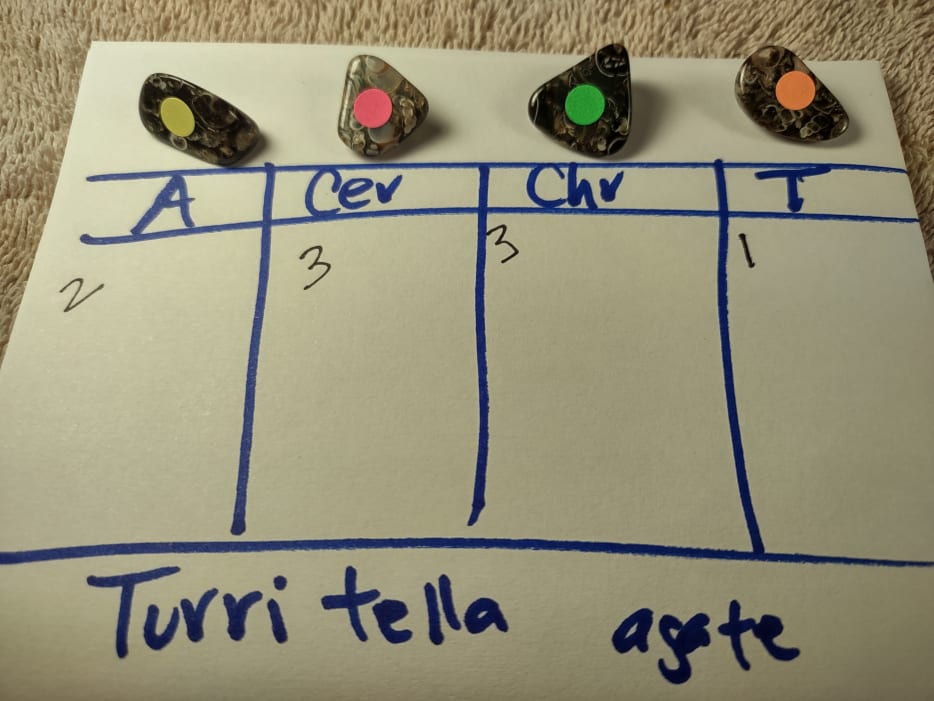   This material was one reason why preparing for this test took 3 years. It takes a long time to tumble 4 pieces of similar size and to not have one that starts going bad with cruddy areas. I'm rather fond of this material for two reasons. First, both words are misnomers. It is neither agate nor are the snails Turritella. They are Elimia. Second, this stuff is uniquely beautiful. Cer was predicted. The T shine I got appears to be perfect and exceeds any shine I ever got before. |
|
Deleted
Deleted Member
Member since January 1970
Posts: 0
|
Post by Deleted on Jul 23, 2022 15:30:03 GMT -5
BobAfter seeing your posts, I was in agreement with HankRocks. It seems TO is almost always the best. Was going to get some but compared the price to AO and Wow! 1lb of AO polish is $5.50. While 1lb of TO polish is $33.00.
|
|
|
|
Post by Bob on Jul 23, 2022 18:29:38 GMT -5
BobAfter seeing your posts, I was in agreement with HankRocks. It seems TO is almost always the best. Was going to get some but compared the price to AO and Wow! 1lb of AO polish is $5.50. While 1lb of TO polish is $33.00. Yea! I got 5 lbs (ouch) a few weeks ago but only because I need to do a 40lb batch soon. But since I reuse polish over and over it does not matter too much over time. I've noticed no decline in alum ox and cerium ox after dozens of uses and hope tin is same. Just today I started a 20lb barrel in tin, using two cups of the new because all my other was on use. |
|
|
|
Post by HankRocks on Jul 23, 2022 19:03:50 GMT -5
Bob After seeing your posts, I was in agreement with HankRocks . It seems TO is almost always the best. Was going to get some but compared the price to AO and Wow! 1lb of AO polish is $5.50. While 1lb of TO polish is $33.00. Yea! I got 5 lbs (ouch) a few weeks ago but only because I need to do a 40lb batch soon. But since I reuse polish over and over it does not matter too much over time. I've noticed no decline in alum ox and cerium ox after dozens of uses and hope tin is same. Just today I started a 20lb barrel in tin, using two cups of the new because all my other was on use. I have been saving and re-using Tin Oxide for over two years now, no noticeable drop off in polishing that I can tell. The only Tin Oxide I lose is with the vibe Lap, whatever sticks to the polished pieces does not get saved. |
|
Deleted
Deleted Member
Member since January 1970
Posts: 0
|
Post by Deleted on Jul 24, 2022 23:27:48 GMT -5
Bob HankRocksI feel like there are a few threads about reclaiming grit but what do you guys do to save yours? Would it work with AO polish?
|
|
Deleted
Deleted Member
Member since January 1970
Posts: 0
|
Post by Deleted on Jul 24, 2022 23:29:01 GMT -5
HankRocksDo you only use TO for polish or other polish too?
|
|
|
|
Post by HankRocks on Jul 25, 2022 2:21:46 GMT -5
Bob HankRocks I feel like there are a few threads about reclaiming grit but what do you guys do to save yours? Would it work with AO polish? Yes is would and does work with AO as that's what I used to polish with before my switch to Tin Oxide. Same method, after rinse into dedicated bucket and let evaporation and settling out do the trick. Once sufficiently dry I reduce the dry cake to powder and save. I also save old slurry using the same method, some for reuse as a slurry starter and for disposal. Obviously when tumbling one creates more slurry than can be re-used. |
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,606
|
Post by jamesp on Jul 25, 2022 14:22:21 GMT -5
Some members have reused AO polish.
It has always stirred up curiosity as to how that works.
Virgin Rock Shed's polish is +/-14,000 grit in size. How big is it after it has been used once or twice ?
Know that 14,000 grit is 1 micron, 60,000 grit is 1/2 micron, 100,000 grit is 1/4 micron.
Say 14,000/1 micron starts at 14,000 and wears down to 60,000/a half micron, how does that affect the rock's polish on the 2nd use ?
Anyone have a good microscope ?
|
|
|
|
Post by HankRocks on Jul 25, 2022 14:50:25 GMT -5
Some members have reused AO polish. It has always stirred up curiosity as to how that works. Virgin Rock Shed's polish is +/-14,000 grit in size. How big is it after it has been used once or twice ? Know that 14,000 grit is 1 micron, 60,000 grit is 1/2 micron, 100,000 grit is 1/4 micron. Say 14,000/1 micron starts at 14,000 and wears down to 60,000/a half micron, how does that affect the rock's polish on the 2nd use ? Anyone have a good microscope ? It seems to make sense to me that 60,000 AO would be a better polish than 14,000 AO. You can probably deduct some of the improvement as there will be the ultra small rubbed off pieces of the polished rock mixed in the saved polish. A microscopic view of some saved AO polish, and Tin Oxide for that matter, would be very enlightening. I know the color of both my saved polishes is several shades darker than unused polish of course some of that could be either left over SiC slurry or even the barrel. Not sure. All I know is both the saved polishes produce great results. |
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,606
|
Post by jamesp on Jul 25, 2022 16:00:13 GMT -5
HankRocks Would skipping from prepolish all the way to 60,000 be a problem ? Just saying that prepolish to 60,000 seems like a big hop. Over the years several members have reused their polish to great success. One of them that comes to mind was carloscinco, he lived near the Rio Grande river about 50 miles downstream from Zapata. He always had show stopper tumbles.
|
|
|
|
Post by HankRocks on Jul 25, 2022 16:10:31 GMT -5
jamesp. I am guessing the jump from Pre-polish to 60,000 would not be a big issue as I am using 500 AO as Pre-polish and after 7 days in Rotary it probably reduced to say 2000 to 3000. I throw one more brain twister to this equation. I save Pre-polish slurry and add it to my Pre-Polish runs(of course I do!!), probably 2 cups worth to a Model B run. Calculate the average grit size now. 
|
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,606
|
Post by jamesp on Jul 25, 2022 16:21:01 GMT -5
jamesp. I am guessing the jump from Pre-polish to 60,000 would not be a big issue as I am using 500 AO as Pre-polish and after 7 days in Rotary it probably reduced to say 2000 to 3000. I throw one more brain twister to this equation. I save Pre-polish slurry and add it to my Pre-Polish runs(of course I do!!), probably 2 cups worth to a Model B run. Calculate the average grit size now.  Good point. No surprise AO 500 wears to 2000-3000 or even 15,000. I mention 15,000 because AO 500 in the Lot-O darn near ends up a wet polish which suggests a 14,000/1 micron finish. All I can say on the twister if the used pre-polish lays down a fine polish itself it must be close to 10,000 to 15,000 grit/1 micron in size. If it leaves a glare-matte finish I'd guess 2000 to 4000 grit size. |
|
|
|
Post by Bob on Jul 26, 2022 13:17:19 GMT -5
Well, it appears my material postings have to stop now. I have got them all done. Last night I assessed where things stood and was embarrassed to find out that I forgot to get two materials into the tin--Exotica jasper and Botswana agate. They have been processed in the other 3 polishes though. It took me examining the photos of all the rocks to figure out for certain what happened. Sure enough they are missing from the group photos after all done. The photo documentation being done has helped me several times to avoid screwups.
That agate was chosen to the the proxy for all agates, so I'm pissed at myself. But some is in a separate batch of tin being polished and by early Sep I will probably be able to post the results. Ditto on the jasper.
In the meantime, I'm going share my general impressions as to what has been learned so far.
Then, I've decided to burnish them all, keeping all 4 polished batches separate. One would not expect burnishing to alter which polish is best, but then again, one doesn't know until after doing something. I'll probably wait until these two materials are done until starting that burnish run.
|
|
Deleted
Deleted Member
Member since January 1970
Posts: 0
|
Post by Deleted on Jul 26, 2022 13:43:59 GMT -5
BobLooking forward to hearing your thoughts and seeing the jasper and agates as well as seeing after burnishing. I’m on the fence about burnishing. I typically don’t do it.
|
|
|
|
Post by Bob on Jul 27, 2022 10:23:06 GMT -5
Well, one of the advantages of making those 34 paper cards corresponding to each material test with the results is I can sort them now by polish type, or by rock material. I will try to categorize results into meaningful material groups that might show trends based upon to what group a material belongs.
First, I want to look at Chromium Oxide (which according to my research is also known as Chromic Oxide, Trivalent Chromium Oxide, Cr203), because it had pretty sorry results. It's reputation in the literature is more or less for materials that:
- are very tough or hard (nephrite, garnet, etc.)
- tend to undercut or contain magnesium or manganese
- are composed of multiple minerals (Rhodonite, Lapis Lazuli, Malachite, Azurite, Unakite)
First, I sorted the cards into a pile of 13 in which Chr placed either 1st or 2nd.
It was 1st in only 3:
1) Hematite, which is a unique metallic material, and it only did barely better than Alum. I'm going to be polishing some psilomelane soon and wonder if it will do best there too.
2) Kambaba jasper, a unique material. None got shiny, but only got satiny, and it only barely did better than Alum.
3) Serpentines: Healerite. This probably didn't even belong in the test at all as it's too soft I think. And all four polishes did the same, all being equally dull so this really doesn't elevate Chr.
As to those other 10 ones where Chr was 2nd, there is a real mix.
Feldspars: For Orthoclase, although it was 2nd, all the other polishes came in equally on 1st, and they all produced a shine but Chr didn't, so being 2nd there matters not. For Amazonite, both Cer and Tin were 1st, and Alum and Chr tied at 2nd, so also no big deal for Chr there.
Rhyolites: For Wonderstone, it was 2nd and so was Cer as a tie.
Cherts: For Mookaite, it was 2nd but so was Alum.
Agates/jaspers: For Pedernal chalcedony, it was 2nd, being behind both Alum and Tin which were both 1st.
Macrocrystalline quartzes: For Green tree "agate", it was 2nd, but tied with Alum.
Fibrous, non-banded chalcedonies: For Chrysoprase, it was 2nd.
Limestones: For Coquina "jasper", it was 2nd but so was Alum and Cer in a 3 way tie, none of which produced a shine.
Feldspathoids: For Sodalite, it was 2nd but both Cer and Tin were 1st.
Amphiboles: For Nephrite, it tied for 2nd with Tin, but both Alum and Cer tied for 1st.
Frankly, in conclusion, it doesn't look to me like Chromium Ox has a lot of use in my rotary tumbling, except for Hematite and Kambaba "jasper". I'd be very interested in knowing if others think the same based upon their own experiences.
I have never tumbled malachite, which is only Mohs 3.5 to 4.0, but I have a few pieces in mid-grind and someday they will get to polish and I will try Chr with them.
|
|
|
|
Post by Bob on Jul 27, 2022 16:15:55 GMT -5
Since it seems like alum and tin were neck-to-neck a lot, and I’ve already covered chrom, I guess next easiest is cerium ox which had 17 of the 34 results as either 1st or 2nd.
It seems to be sold for tumbling in one of two substypes. There is tan/pink optical grade, also called Optical Grade Cerium, Cerium IV Oxide, Optician's Rouge, 90% Pure Cerium which is generally what we are buying. There is also a white faceting grade, also called Opaline, French Cerium, Super Cerium which is considered overkill for tumbling. It’s reputation in the literature is more or less:
- For Agate, quartz, crystal quartz, Amethyst, Jasper, Petrified Wood, Feldspar, Glass, Obsidian.
- Was preferred until Alum replaced due to cost. Some still prefer for Feldspar and Obsidian.
- Not good for soft material or material that undercuts.
Since I had used only it for years, I was kinda holding out for my old friend to do well in at least some things.
It was 1st in 5.
1) Feldspathoids: For Sodalite, but tied with Tin.
2) Feldspars: For Orthoclase, but tied with Tin. Amazonite, ditto.
3) Serpentines: Healerite. This probably didn't even belong in the test at all as it's too soft I think. And all four polishes did the same, all being equally dull so this really doesn't elevate Cer.
4) Amphiboles: For Nephrite, it tied with Alum.
So it accomplished nothing that Tin (or in one case Alum) didn’t do as well. Because of that, I don’t think there is any use in even mentioning the instances in which it came in 2nd.
Frankly, in conclusion, it doesn't look to me like Cerium Ox has any use in my rotary tumbling at all, because I anticipate mostly using Tin or Alum going forward. This was unexpected. I'd be very interested in knowing if others think the same based upon their own experiences.
I’m also sad that I have a lot of it on hand, probably between 5 and 10lbs and much of it unused, so if anyone wants to buy some from me please let me know. Not that my results have actually encouraged any demand for it!
|
|
|
|
Post by Bob on Jul 28, 2022 9:55:26 GMT -5
Apparently, I need to declare that comparison above for black jasper as invalid. In the photo, there are 2 flat pieces that look just like stream gravel. In fact, this is from large bags of this I used to buy on eBay from a seller in California and I think he told once it was stream gravel. I used it for filler and about 10% came out pretty decent in polish and I have a large bowl of the stuff. The exact identify of this isn't certain but I think it is probably basanite, or maybe tiny pieces of basalt.
The 2 more three-dimensional pieces are from a batch of purchased black jasper I bought a couple of years ago. Comparing the feel and appearance of these to other colored jaspers I have purchased, they do seem like jasper.
Somehow, I accidentally combined these when doing the comparison. In looking at their surfaces with a 10x lens, I do believe they are not the same material. The pieces of what I'm calling basanite are definitely grey. The 2 pieces of jasper are black or very dark grey just shy of black. The jasper also has a tighter surface with fewer microcrevices (not visible to the naked eye).
|
|
|
|
Post by Bob on Jul 28, 2022 10:29:21 GMT -5
Since several people commenting and I seem to think tin is going to be the big winner here, I will save it for last and comment on Aluminum Oxide now.
There are at least 6 variations of this polish being sold for rock/gem tumbling or lapping. Some is only for flat lapping. There are only two that seems applicable here and for which I was also able to find some decent application info in the literature:
Micro-Alumina (aka Alundum or Alodur), which is in the subcategory of fused alumina, is the general product being sold to rock tumblers:
- Agate, Jasper, Quartz, etc., almost everything. Preferred now over Cerium due to cost.
Linde A (Sapphire Powder), which is in the subcategory of levigated alumina:
- Expensive, so polish with something else first. Good for tourmaline, Beryl, Garnet, Topaz, Peridot. I have never bought any or used it.
Of the 34 cards, this polish came in 1st nine times.
It tied with tin for:
1) Feldspars: Orthoclase.
2) Serpentines: Healerite.
3) Cherts: Vanport chert.
4) Prehnite, a unique material.
5) Dalmatian stone, a unique material.
6) Fibrous, non-banded chalcedonies: For Chrysoprase (very low quality), Pedernal chalcedony, and Moss “agate”.
It only did better than tin once:
7) Amphiboles: Nephrite, it tied with Cer, both of which edged out Tin a tiny bit.
So it accomplished nothing that Tin didn’t do as well, except for Nephrite. Because of that, I don’t think there is any use in even mentioning the instances in which it came in 2nd.
Frankly, in conclusion, it doesn't look to me like Aluminum Ox has much use in my rotary tumbling at all, because tin is 1st on so many things and alum usually does no better. This was extremely unexpected as that was most of what I’d been using for the last 4-5 years. I'd be very interested in knowing if others think the same based upon their own experiences. It’s so much cheaper though than tin, that if I was unable to recycle tin and just keep using it, I would probably use alum most of the time instead.
|
|
|
|
Post by Bob on Jul 29, 2022 16:08:31 GMT -5
Well, here we go on the last polish, Tin Oxide, also known from my research as Tin Dioxide, Stannic Oxide, Cassiterite, "Flowers of Tin".
The literature seems to recommend it for:
- Agate, Quartz, Glass, Obsidian, Ruby, Sapphire, Opal, Topaz, Beryl, Coral, Amber, Jadeite, Jasper, Petrified Wood. Due to cost, many users use only for soft such as Calcite, Rhodochrosite, Malachite.
When I sorted out the results cards for instances in which this polish was 1st or 2nd, only two cards remained not in that group (Hematite, and Kambaba “jasper”). The only one for which tin came in 2nd was Nephrite being edged out almost slightly by alum and cer. But the pile of results cards for which tin came in 1st and nothing tied with it was huge, 20 of the 33! (33 not 34 because I have invalidated the black jasper test as of a day or two ago.) It had something else tie with it for 1st 10 times. I think it will be helpful for potentially spotting any material groups for which tin is outstanding if I combine them below:
1) Unakite, a unique material, uncontested 1st.
2) Epidotes (which is both the name of a mineral group as well as an individual mineral), Epidote, uncontested 1st.
3) Rhodonite, a unique material, uncontested 1st.
4) Jaspers, for Spider-woman jasper (which I suspect is really a rhyolite), and Picture Jasper, both uncontested 1st.
5) Quartzites, for Aventurine, and for uncategorized Quartzite, both uncontested 1st.
6) Tiger’s eye, a unique material, uncontested 1st.
7) Lapis lazuli, a unique material, uncontested 1st.
8) Petrified organic material, for “Turritella agate”, uncontested 1st.
9) Cherts: For Boley “agate”, and for Mookaite, both uncontested 1st. For Vanport chert, tied with alum for 1st.
10) Macrocrystalline quartzes: For Green tree "agate", uncontested 1st.
11) Feldspars: For Labradorite, and for Moonstone, both uncontested 1st. For Orthoclase, was a 3 way tie with alum and cer for 1st. For Amazonite, tied with cer for 1st.
12) Limestones: For Coquina "jasper", uncontested 1st.
13) Rhyolites: For Wonderstone, uncontested 1st.
14) Glasses: for Obsidian, and for man-made Glass, both uncontested 1st.
15) For Dalmatian stone, a unique material, tied with alum for 1st.
16) Feldspathoids: For Sodalite, tied with cer for 1st.
17) Fibrous, non-banded chalcedonies: For Chrysoprase, uncontested 1st. For Chrysoprase (low quality), Pedernal chalcedony, and Moss “agate”, tied with alum for 1st.
18) Prehnite, a unique material, tied with alum for 1st.
19) Serpentines: Healerite. Tied with all the other polishes—equally lousy results.
Before doing this test, I had only used tin ox once just to test it, and at the moment don’t remember what impression it made on me. But it has certainly made an impression on me in this test.
I’m going to be going forward using tin a lot, and for a lot of different materials--it appears to be a first choice polish except for Hematite and Kambaba jasper both of which need chromium. For Nephrite, cerium and alum outdid it by such a tiny bit that it's almost undetectable, and since no polish produced better than a satin sheen it hardly matters. If you have similar or different opinions about it, I would enjoy reading them.
I’m a little exhausted by all the work this has been. No doubt in all this there has been an error or two, but I’ve tried hard to avoid them. One reason I took all those photos of the methodology, was to be as transparent as possible in everything done so you might have faith in how it was done.
There are a few big overview comments I want to make after thinking about them a little longer.
|
|