|
|
Post by kk on Aug 4, 2014 6:12:34 GMT -5
Got a FA that peels like an onion and crumbles too.   Started out with a good 100gr and got now down to about 70ct without finding god fire. But what I did find is that intense orange red color. Its not from Limonite as it should be in fire agate, but its also not really in the chalcedony/sard itself. When peeling off, the pieces are clear. So have no idea where the color actually comes from. Here is after more than half was cut away from the original piece.  And yesterday  Still got to try harder to turn that nob at the bottom into a visible bug. But I'm afraid things might break away at any moment. Another experiment that draws to its close is polishing a shell we found recently.  Hope to find someone who can cast me a fellows head and hands to mount into the opening. |
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,603
|
Post by jamesp on Aug 4, 2014 17:29:00 GMT -5
is it similar to this color Kurt ? (swamp agate)  |
|
|
|
Post by kk on Aug 4, 2014 19:00:11 GMT -5
Looks very similar. Mine seems to be illuminated from within. Very strange yet strong colour There are some fire agate bubbles underneath, but by what I can make out, they appear green.
|
|
gemfeller
Cave Dweller 
Member since June 2011
Posts: 4,059 
|
Post by gemfeller on Aug 4, 2014 20:13:08 GMT -5
Kurt, iron in some form is the coloring agent (chromophore) in both brown sard and reddish carnelian chalcedonies. It could be the thin sections that appear clear to you are actually lightly tinted brown/red but it isn't apparent. According to physics, color intensifies the further a light wave travels within a substance. Faceters take advantage of that by cutting deeper pavilions in light-hued gems to improve color. Everything I've read attributes the color-play layers in fire agate to an iron mineral also: goethite is most commonly mentioned. A recent post by Danny Lopacki seemed to claim otherwise, based on research by one of his friends. But he said he couldn't reveal the information without permission, so we're still waiting for that.
I'm familiar with the fire agate structure you mention. I have a few pieces from an undisclosed location made of very thin rounded layers that peel like an onion. It has POC but is the very devil to sand and polish in one uniform surface. All fire agate is botryoidal in form but what causes the thin, fragile layers vs. solid thicker layers is a mystery to me. I've always assumed F.A. was formed from silica dissolved by hot water and deposited slowly in layers like cave stalagmites. I speculate that at some point the source of iron in the feedstock was depleted and the familiar white iron-free "chalcedony roses" formed at the end of the process. But that's all based on deductive reasoning. The really odd part is that so far (to my knowledge) no fire agate has been found anywhere in the world except the Sonoran Desert of the U.S./Mexico. Why it would form only there and not in worldwide volcanic/hot spring areas is an abiding mystery to me.
If you'll send me a wax carving of the figure you want for the shell I'll see to the casting. |
|
gemfeller
Cave Dweller 
Member since June 2011
Posts: 4,059 
|
Post by gemfeller on Aug 4, 2014 20:23:44 GMT -5
Kurt, a second reading of your post suggests you're referring to what causes the intensity of the reddish color, not the cause of the color itself. I've observed the same phenomenon and can only speculate that light reflected from the mirror-like color layers below may intensify the hue somehow. However James's specimen achieves the same "glow" with no reflecting layer below. Interesting question.
|
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,603
|
Post by jamesp on Aug 4, 2014 20:52:27 GMT -5
|
|
|
|
Post by kk on Aug 5, 2014 8:12:48 GMT -5
Kurt, a second reading of your post suggests you're referring to what causes the intensity of the reddish color, not the cause of the color itself. I've observed the same phenomenon and can only speculate that light reflected from the mirror-like color layers below may intensify the hue somehow. However James's specimen achieves the same "glow" with no reflecting layer below. Interesting question. Yeah, but nevertheless, your explanations and assumptions are just as interesting to read. Yes have seen the post by Lopaki, but he is promising this article since years.   So don't hold your breath yet.   Whatever the research yielded might be considered controversial to the point where his friend is not yet willing to come forward with. Will see and wait. In the meantime, I hope "Silverfox" will get her feet on the ground again and pursue her "Meteor" theory. This piece is now the fifth from that particular location that has yielded two for a garden-toss, two with very weak color and peeling, and one with very intense oily colors, but very dark so its hard to see them. All of them, where interesting by themselves, featuring strange compositions and Carnelian features. I always presumed that Carnelian does not have growth-rings? Both peelers have them in the most intense parts (Lizard as well as this one). Here is a better look, hope it will embed here, otherwise it will link you to youtube.com to see the video <iframe width="420" height="315" src="//www.youtube.com/embed/r1lvP8ww7NI" frameborder="0" allowfullscreen></iframe> Best regards, Kurt |
|
|
|
Post by kk on Aug 5, 2014 8:22:55 GMT -5
Yeah James you are the lucky guy who finds all sorts of strange things in your corals. But the first example you showed, is pretty much the best match to what I see here.
|
|
gemfeller
Cave Dweller 
Member since June 2011
Posts: 4,059 
|
Post by gemfeller on Aug 5, 2014 17:18:10 GMT -5
Kurt, from my reading I don't think even so-called experts can distinguish totally between chalcedony and agate. As in your case, banded material often occurs right alongside pure unbanded chalcedony in the same stone. There are long-winded explanations for why the banding occurs but I'll avoid them for the moment. These images show a couple of rough fire agates with very friable layering, the most extreme I've ever seen. I have several pieces and they're all formed the same way. The material has POC but I couldn't get the lighting right to show it. I got it from a man I met when I was visiting Diamond Pacific in Barstow, CA but he wouldn't tell me the source. I'm glad I didn't buy more because the layering makes it very difficult to cut solid stones. The POC is a little odd too: more like pale interference colors from an oil slick on water than usual F.A. colors. One more F.A. oddity to add to the list.  [/URL]  [/URL |
|
|
|
Post by kk on Aug 5, 2014 19:05:48 GMT -5
Yeah, Dean send me something like the recently as a freebie from dear creek to try out. Very odd colour, but fascinating. Very thin layers, hope I will be able to preserve some.  |
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,603
|
Post by jamesp on Aug 5, 2014 19:08:03 GMT -5
The stuff I find does have layers in some cases, mostly at the outer most layers.
Usually it is hard and takes tumbling, but in no respect compares to Fire Agate.
|
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,603
|
Post by jamesp on Aug 5, 2014 19:42:29 GMT -5
Will be interesting to see the shell carved.
|
|
TByrd
fully equipped rock polisher
   Have you performed your random act of kindness?
Have you performed your random act of kindness?
Member since December 2010
Posts: 1,350
|
Post by TByrd on Aug 5, 2014 20:53:26 GMT -5
Those are gorgeous rocks. Very very beautiful.
|
|
|
|
Post by kk on Aug 6, 2014 0:05:36 GMT -5
Will be interesting to see the shell carved. Nahhh , the shell is going to be fully polished, then capped in silver and a figure set into the opening. |
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,603
|
Post by jamesp on Aug 6, 2014 7:00:43 GMT -5
Will be interesting to see the shell carved. Nahhh , the shell is going to be fully polished, then capped in silver and a figure set into the opening. Cool, curious to see what the surface looks like. Totally interesting patterns, and it looks thick. Shells have some great colors and patterns. |
|
|
|
Post by kk on Aug 7, 2014 7:17:05 GMT -5
Kurt,
If you'll send me a wax carving of the figure you want for the shell I'll see to the casting. No can do. I have never done wax.   In this case, it needs precision to fit, something I have to ask someone else to do. I'm not suited for that job.   |
|
|
|
Post by 1dave on Aug 7, 2014 8:56:57 GMT -5
There is so much we do not know about silicon and iron transition points. especially in the cooler crystallizing areas. 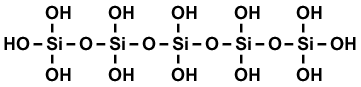 Chalcedony Molecule The water in the chalcedony molecule seems to cause it to alternate between needle quartz crystals and botryoidal forms. The OH attachments seem delicate. All forms of iron "rust" into limonite that is easily carried and deposited by water. As limonite dries out, it crystallizes into goethite. I don't know how specular hematite is formed, but I have long suspected it for the color play in fire agate, but this from Mindat makes me wonder about the role of phosphates. "Rainbow Hematite" from Brazil. Three special formation conditions would account for its scarcity. |
|
gemfeller
Cave Dweller 
Member since June 2011
Posts: 4,059 
|
Post by gemfeller on Aug 7, 2014 10:27:32 GMT -5
There is so much we do not know about silicon and iron transition points. especially in the cooler crystallizing areas. So true! Very little research money is dedicated to the topic because of the relatively low value of quartzes. |
|
gemfeller
Cave Dweller 
Member since June 2011
Posts: 4,059 
|
Post by gemfeller on Aug 7, 2014 13:41:32 GMT -5
Dave: back to chalcedony formation. I've often wondered what role moganite/chalcedony polymorphism plays in determining how cryptocrystalline quartzes form. I wonder if that could be part of the explanation for fire agate's structure, with moganite's monoclinic crystallization affecting how F.A. is deposited.
|
|
|
|
Post by 1dave on Aug 7, 2014 15:30:24 GMT -5
Dave: back to chalcedony formation. I've often wondered what role moganite/chalcedony polymorphism plays in determining how cryptocrystalline quartzes form. I wonder if that could be part of the explanation for fire agate's structure, with moganite's monoclinic crystallization affecting how F.A. is deposited. Fifty years ago I was deeply involved with understanding high temperature silicas. I had two brothers working for Texas Instruments during the microchip revolution and had access to information on their experiments. No one was interested in room temperature and cooler. Then it seams all the scientists died off and the new generation decided we now know everything. Scott - @shotgunner is doing some experimenting. He sent me information on how Gilson made opals. |
|