|
|
Post by 1dave on Sept 30, 2017 7:36:58 GMT -5
jamesp I got to wondering if ph has any effect in tumble polishing. There are three kinds of feldspar, so there are three kinds of clay. 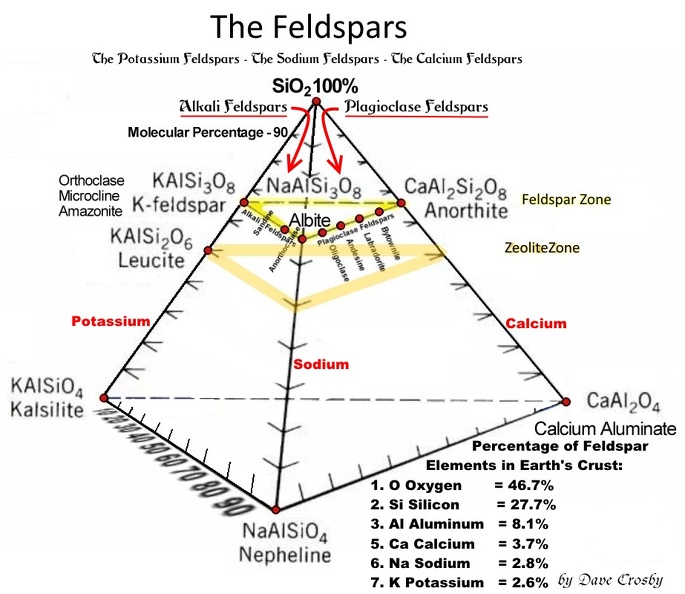 Calcium, Sodium, Potassium. Acid dissolves calcium and deposits silica. Would an acidic slurry deposit silica and fill in scratches on agate? Perhaps add a little vinegar? Base dissolves silica and deposits calcium. Would it help polish calcite? What about other soft stones?
|
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,604
|
Post by jamesp on Sept 30, 2017 10:47:14 GMT -5
No doubt Dave. Some of the combinations of rocks and slurry and water are going to have ph reactions. Not sure it matters other than blowing the cap off of a sealed rotary barrel. Creating gas... A cheap swimming pool ph tester would do the trick. Two items to measure. Rocks and water. Water easy to measure. Rocks not so easy being a solid. Coarse grind is when ph reactions occur. When you are cutting meat off the rocks. Not finishing steps. Never had gas during 220-500-1000-14,000 in rotary. No meat removal. Water has a lot of variation in ph. Ever seen the reaction of baking soda and vinegar ? Gas galore. Just curious what types of ph cocktails are being generated in the tumbler. ph reactions are most common.   Dasani is acid 3.04  , Essentia is alkaline 9.26. Wide range, about as wide a range as baking soda and vinegar. Am I missing something ? Guaranteed, tumbling alkaline lime bearing type agates with Dasani ph 3.04 acid water is going to generate gas. Got to. Does not make sense Dasani is ph 3. But water does vary greatly. Acid water with acid rocks or alkaline water with alkaline rocks makes sense. Not sure how you would measure the ph of rocks. Unless you measure their slurry, and then you need to know the ph of the water you mixed with the rocks. 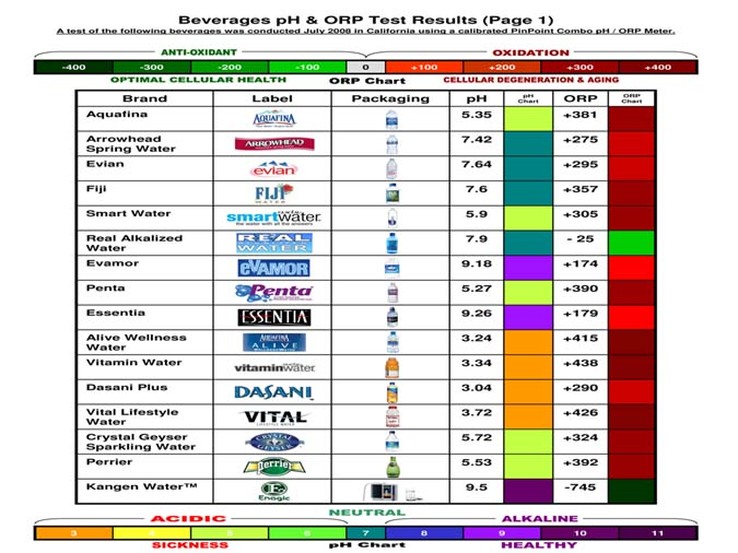  |
|
|
|
Post by pauls on Sept 30, 2017 15:26:23 GMT -5
Interesting.
I never have trouble with gassing, always actually pulling a vacuum inside my barrel.
I am tumbling Agate Creek Queensland Australia Agates almost exclusively.
Some of the Agates I collect have Calcite and/or Aragonite inside vuggs, some have Agate pseudomorphs after Calcite or Aragonite, some have yellow flecks of Calcite (presumably) included that make the Agates fluoresce under UV. So presumably the rock is basic. I don't tumble the Calcite if I can help it.
The water I use is country rainwater.
I am tumbling in a steel LP gas tank. A 4.5Kg tank.
I have tumbled in a highly caustic slurry by adding Caustic Soda.
My thinking was that the caustic might help the slipperyness of the slurry, chemically remove some Agate edges and dissolve rock powder out allowing more contact with Silicon carbide grit.
It worked to a certain extent, I'm not sure that it did much of anything to the actual rocks, but it certainly dissolved the rock powder from the slurry.
I didn't keep it going as it was just too dangerous working with a highly caustic slurry plus if you let the slurry dry out exposed to the air it would absorb Carbon dioxide and the Sodium Silicate would harden to a brick.
I might have to try adding a bit of acid to one of my plastic barrels. Prepare for barrel explosions I guess.
|
|
|
|
Post by 1dave on Sept 30, 2017 18:50:12 GMT -5
I worked on a job where there was a caustic soda spill - ate up some workers leather boots!
|
|
Deleted
Deleted Member
Member since January 1970
Posts: 0
|
Post by Deleted on Sept 30, 2017 21:13:25 GMT -5
Interesting. I never have trouble with gassing, always actually pulling a vacuum inside my barrel. I am tumbling Agate Creek Queensland Australia Agates almost exclusively. Some of the Agates I collect have Calcite and/or Aragonite inside vuggs, some have Agate pseudomorphs after Calcite or Aragonite, some have yellow flecks of Calcite (presumably) included that make the Agates fluoresce under UV. So presumably the rock is basic. I don't tumble the Calcite if I can help it. The water I use is country rainwater. I am tumbling in a steel LP gas tank. A 4.5Kg tank. I have tumbled in a highly caustic slurry by adding Caustic Soda. My thinking was that the caustic might help the slipperyness of the slurry, chemically remove some Agate edges and dissolve rock powder out allowing more contact with Silicon carbide grit. It worked to a certain extent, I'm not sure that it did much of anything to the actual rocks, but it certainly dissolved the rock powder from the slurry. I didn't keep it going as it was just too dangerous working with a highly caustic slurry plus if you let the slurry dry out exposed to the air it would absorb Carbon dioxide and the Sodium Silicate would harden to a brick. I might have to try adding a bit of acid to one of my plastic barrels. Prepare for barrel explosions I guess. I too notice a vacuum on my barrels. Cold day fill/seal hot day clean out. Still the lid is sucked on. No explanation. Just observation. I'm using tap water, kitty litter clay and silica rocks. Never borax. I still don't have polished stuff........ Sorry not much help..... |
|
|
|
Post by 1dave on Sept 30, 2017 22:25:16 GMT -5
Interesting. I never have trouble with gassing, always actually pulling a vacuum inside my barrel. I am tumbling Agate Creek Queensland Australia Agates almost exclusively. Some of the Agates I collect have Calcite and/or Aragonite inside vuggs, some have Agate pseudomorphs after Calcite or Aragonite, some have yellow flecks of Calcite (presumably) included that make the Agates fluoresce under UV. So presumably the rock is basic. I don't tumble the Calcite if I can help it. The water I use is country rainwater. I am tumbling in a steel LP gas tank. A 4.5Kg tank. I have tumbled in a highly caustic slurry by adding Caustic Soda. My thinking was that the caustic might help the slipperyness of the slurry, chemically remove some Agate edges and dissolve rock powder out allowing more contact with Silicon carbide grit. It worked to a certain extent, I'm not sure that it did much of anything to the actual rocks, but it certainly dissolved the rock powder from the slurry. I didn't keep it going as it was just too dangerous working with a highly caustic slurry plus if you let the slurry dry out exposed to the air it would absorb Carbon dioxide and the Sodium Silicate would harden to a brick. I might have to try adding a bit of acid to one of my plastic barrels. Prepare for barrel explosions I guess. I too notice a vacuum on my barrels. Cold day fill/seal hot day clean out. Still the lid is sucked on. No explanation. Just observation. I'm using tap water, kitty litter clay and silica rocks. Never borax. I still don't have polished stuff........ Sorry not much help..... Well, thanks for chiming in. There has to be some interesting chemistry going on in those barrels! |
|
|
|
Post by youp50 on Oct 1, 2017 3:35:47 GMT -5
The chemical depositions that move silica and calcite occur on over a much longer period of time than the mechanical forming occurring in tumbler barrels. I would tend to use a chemical that would aid in the removal of material, much like what pauls did.
Having dealt with acid and caustic reactions to my flesh as a young man, I am good with the hard water around here.
Your skin is quite an effective protector against pH extremes, breaks in your skin is where the problem is.
|
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,604
|
Post by jamesp on Oct 1, 2017 6:45:19 GMT -5
Acid/alkaline water Acid/alkaline rocks The water is easy to test. It is a constant at start up. Testing the slurry after a day or two in coarse grind should give you and idea of which direction the ph is headed. There is so many combinations with rocks. I experimented with hydrated lime as a slurry thickener lol. ph 12. Had to wear gloves. Totally caustic and would flat out eat your skin. Same ph as wet concrete. Made a nice creamy slurry. But not worth the hassle of cooking hands. I never encountered gas, or vacuum. But again, that all depends on the rock's composition. www.flickr.com/photos/67205364@N06/albums/72157648702228508Settlement after 48 hours  Creamy and tacky slurry  |
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,604
|
Post by jamesp on Oct 1, 2017 6:47:34 GMT -5
Interesting. I never have trouble with gassing, always actually pulling a vacuum inside my barrel. I am tumbling Agate Creek Queensland Australia Agates almost exclusively. Some of the Agates I collect have Calcite and/or Aragonite inside vuggs, some have Agate pseudomorphs after Calcite or Aragonite, some have yellow flecks of Calcite (presumably) included that make the Agates fluoresce under UV. So presumably the rock is basic. I don't tumble the Calcite if I can help it. The water I use is country rainwater. I am tumbling in a steel LP gas tank. A 4.5Kg tank. I have tumbled in a highly caustic slurry by adding Caustic Soda. My thinking was that the caustic might help the slipperyness of the slurry, chemically remove some Agate edges and dissolve rock powder out allowing more contact with Silicon carbide grit. It worked to a certain extent, I'm not sure that it did much of anything to the actual rocks, but it certainly dissolved the rock powder from the slurry. I didn't keep it going as it was just too dangerous working with a highly caustic slurry plus if you let the slurry dry out exposed to the air it would absorb Carbon dioxide and the Sodium Silicate would harden to a brick. I might have to try adding a bit of acid to one of my plastic barrels. Prepare for barrel explosions I guess. I too notice a vacuum on my barrels. Cold day fill/seal hot day clean out. Still the lid is sucked on. No explanation. Just observation. I'm using tap water, kitty litter clay and silica rocks. Never borax. I still don't have polished stuff........ Sorry not much help..... I get more vacuums than positive pressure Scott. |
|
|
|
Post by 1dave on Oct 1, 2017 6:58:40 GMT -5
The chemical depositions that move silica and calcite occur on over a much longer period of time than the mechanical forming occurring in tumbler barrels. That is the "scientific" opinion. When temperature, pressure, ph are just right, things happen rapidly! Many minerals have multiple transition points. When one of them is met, SHAZAM! the change from one crystallographic form to another happens! Water and silica go through numerous transitions separated only by many degrees in temperature. I have an Admiral Fitzroy's Storm Glass Weather Instrument on my desk and often watch it go through all kinds of structural changes. www.thoughtco.com/make-fitzroys-storm-glass-609443/stormglass-56a129775f9b58b7d0bca129.jpg) |
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,604
|
Post by jamesp on Oct 1, 2017 7:36:18 GMT -5
ph has to play a big role in gas build up in a sealed rotary barrel. If you have ever tumbled concrete you will find the slurry is very alkaline. Even if you add just 20% concrete chunks mixed with agate. That 20% concrete will often create pressure in a sealed rotary. Concrete chunks are like adding alkaline pills to the mix. Tumbling brass, copper and stainless steel also creates positive gas pressure(in my case). My water is ph 6. One solution to tumbling high gas materials is simply using a slant rotary tumbler where the contents do not need to be sealed.  |
|
Deleted
Deleted Member
Member since January 1970
Posts: 0
|
Post by Deleted on Oct 1, 2017 9:41:01 GMT -5
The chemical depositions that move silica and calcite occur on over a much longer period of time than the mechanical forming occurring in tumbler barrels. I once made glass clear agate in under an hour. It was a failed experiment in that my method would not allow for production quantities reliably. But as proof of concept I did have cabochon sized agate in 40 minutes or so. |
|
|
|
Post by 1dave on Oct 1, 2017 9:48:03 GMT -5
The chemical depositions that move silica and calcite occur on over a much longer period of time than the mechanical forming occurring in tumbler barrels. I once made glass clear agate in under an hour. It was a failed experiment in that my method would not allow for production quantities reliably. But as proof of concept I did have cabochon sized agate in 40 minutes or so. That process could be used to fill pits and heal fractures in agate slabs! |
|
Wooferhound
Cave Dweller  Lortone QT66 and 3A
Lortone QT66 and 3A
Member since December 2016
Posts: 1,432 
|
Post by Wooferhound on Oct 1, 2017 10:00:13 GMT -5
I have tried polishing some hard limestone with mixed results, mainly dull looking. I wonder if adjusting the PH would help ? jamesp I got to wondering if ph has any effect in tumble polishing. There are three kinds of feldspar, so there are three kinds of clay. |
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,604
|
Post by jamesp on Oct 1, 2017 10:47:08 GMT -5
I have tried polishing some hard limestone with mixed results, mainly dull looking. I wonder if adjusting the PH would help ? jamesp I got to wondering if ph has any effect in tumble polishing. There are three kinds of feldspar, so there are three kinds of clay. Hard limestone can be variable depending on silicification. I suppose some real hard cherts are silicified limestone. I would not think ph is going to effect the abrading process. Abrasion being totally mechanical. Or supposed to be. Muriatic acid will sure eat the some limestone pockets out of agate. But that is some serious acid. Certainly no one is going to tumble rocks in it. |
|
|
|
Post by 1dave on Oct 1, 2017 13:04:06 GMT -5
I think tumbling in strong solutions on either end would be more disastrous than helpful - to rocks and equipment.
|
|
|
|
Post by amygdule on Oct 1, 2017 15:50:59 GMT -5
Mud in one end, three hours @1500 degrees later, out comes the nice uniform pebbles. Interesting Chemistry as a Lime Kiln Operator. 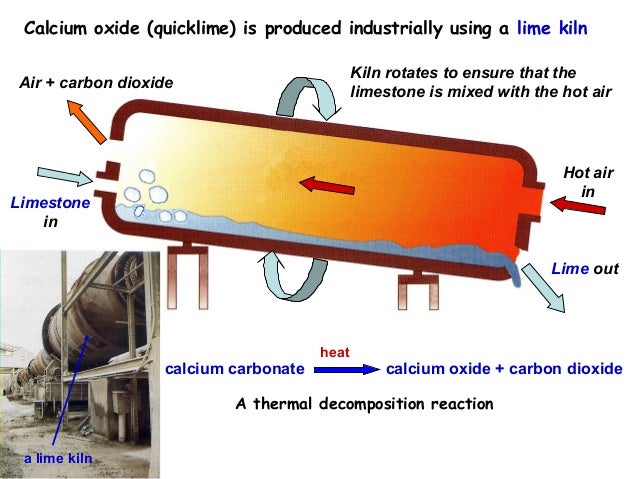  |
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,604
|
Post by jamesp on Oct 1, 2017 18:00:46 GMT -5
Mud in one end, three hours @1500 degrees later, out comes the nice uniform pebbles. Interesting Chemistry as a Lime Kiln Operator.   Now that's a tumbler. Cool process equipment. MASSIVE |
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,604
|
Post by jamesp on Oct 1, 2017 18:35:34 GMT -5
I think tumbling in strong solutions on either end would be more disastrous than helpful - to rocks and equipment. Let the mechanics do the work ? YES. It is complicated enough without involving chemistry. Unfortunately the rock does grind into particles perfect for reactions. Not much can be done about that. No telling what kind of brews have been amalgamated in the ole rotary. i know that my coral and Rio agates-jaspers-and pet wood makes a plant friendly slurry. As far as the coral's high limestone content I would assume it is the lime added at a near chelated state from the fine grinds. Ag lime. Ag lime is faster acting as it is finer. 1 inch pebbles take a long long time to raise ph of soil. ag lime is normally 30-90 mesh, it varies. For maintaining a healthy pasture in most of the east 1 to 4 tons of Ag lime is spread every 3 to 5 years per acre. |
|
|
|
Post by youp50 on Oct 1, 2017 21:45:44 GMT -5
I have worked on line kilns, it sucks. If you get lucky enough to work on one in a paper mill, there are usually other processes nearby that also offer poisonous fumes.
|
|