Post by 1dave on Dec 16, 2016 17:45:15 GMT -5
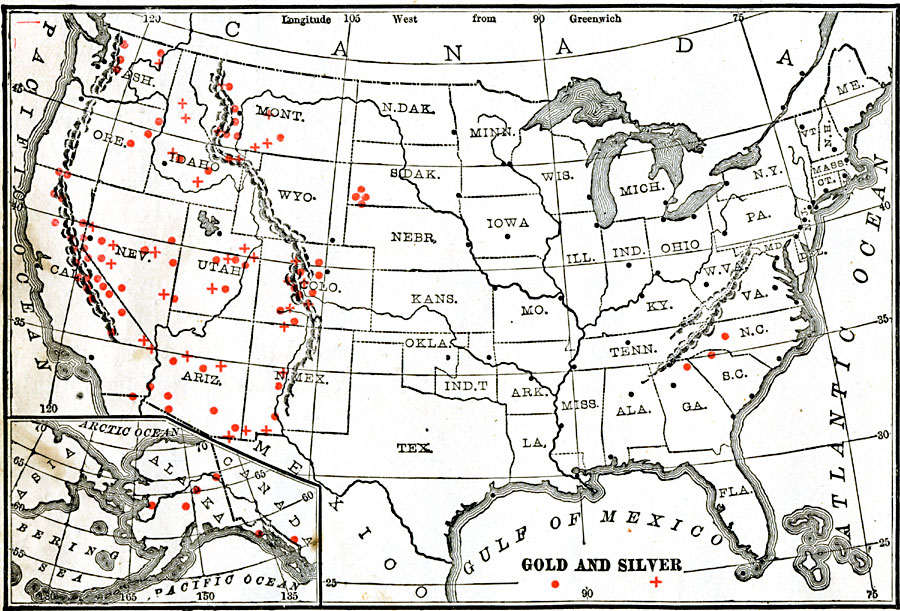
Gold and Silver Mines O = Gold, + = Silver
When someone discovers a valuable mineral, pretty soon they need to know what the area is worth.
Should they spend a lot of time and money developing the property, or will it cost more than can be produced?
These questions opened Assay Offices all over the west with each new discovery of more Gold and Silver mines.
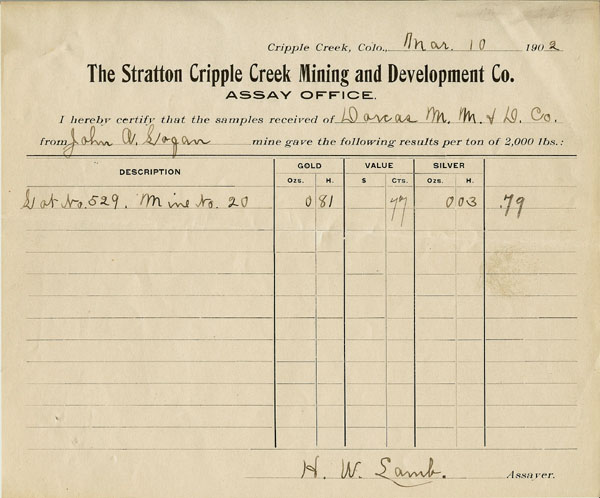
Assay Certificate
What is assaying and how do you go about doing it?
NOTE: If you are serious about this subject you may want to study:
Notes on assaying and assay schemes -1876.
Lab instructions - 1913
Or join 911 Metallurgist.

Old Assay Office
The art of assaying is a branch of analytical chemistry in connection with mining and metallurgy. It's object is to obtain the value of a stated quantity (usually in avoirdupois ton) by determining the value of a small representative sample.
- J. Reginald Smith "Assaying For Everyone" 1902
(Printed on Demand)
- J. Reginald Smith "Assaying For Everyone" 1902
(Printed on Demand)

Modern Assaying: A Concise Treatise, Describing Latest Methods and Appliances (Classic Reprint) (Paperback) - by J. Reginald Smith (Author) Paperback $10.57
So assaying is an attempt to determine the amount and value of a metal in a field, stream bed, mine, or vein.
I think of the Assay Process as functioning like a pinhole camera where:
1. the subject is the mine, 2. the pinhole is the sample, and 3. the image is the emerging estimated amount and value.

A. A rough assay can be done by crushing ore samples and panning the results. With some experience and a careful examination of the amount of gold present, it is possible to make decent visual estimates of the amount of free gold in the ore.
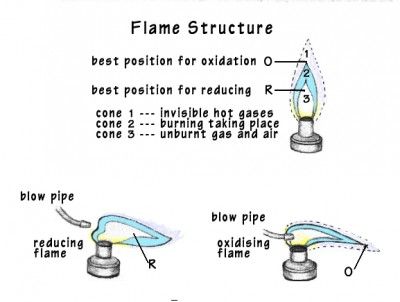
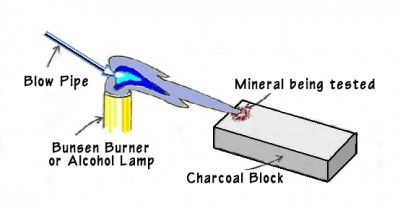
B. Assaying by the blow-pipe furnace is another inexpensive path.
Click here for a list of Assay Tools:
ASSAY PREPARATION
Step 1. Sampling

The first step in assaying is to obtain a sample, and it is all important that the portion selected should be representative of the entire quantity. No special pieces should be picked from the bulk, but the desired quantity is taken indiscriminately from the entire original parcel.
Accurate samples which reflect the true average of the material must be collected. If the sample does not reflect the average nature of the material, no amount of technical accuracy in processing the sample will provide a correct result.
Without a fair sample the assay is useless!
Step 2. Crushing -

Grind or break the entire quantity of the sample to as near as possible a uniform size.
Step 3. Quartering
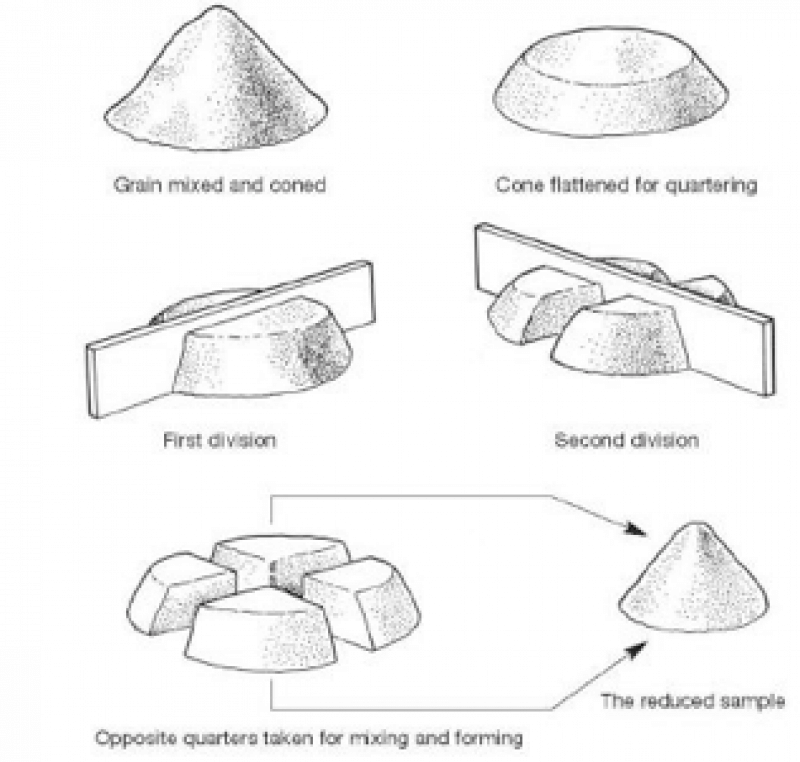
Now thoroughly mix by stirring and rolling the crushed ore on a large mixing sheet from end to end of the sheet and then from side to side.
Divide into four equal divisions by making a line through the center of the entire sample, and then another line through the center of the sample at right angles to the first line. take the two divisions that are diagonally opposite and discard the balance. Repeat this operation after thoroughly remixing the diagonal portions, and continue doing so until the final remaining two diagonally opposite divisions make such a quantity will furnish pulp (powder) for several assays of the same sample. Care must be used in this operation as the accuracy of the assay depends largely on this.
Divide into four equal divisions by making a line through the center of the entire sample, and then another line through the center of the sample at right angles to the first line. take the two divisions that are diagonally opposite and discard the balance. Repeat this operation after thoroughly remixing the diagonal portions, and continue doing so until the final remaining two diagonally opposite divisions make such a quantity will furnish pulp (powder) for several assays of the same sample. Care must be used in this operation as the accuracy of the assay depends largely on this.
Step 4. Pulverizing or Pulping

buck-board and muller
It is now necessary to reduce the remaining sample to a fine powder usually called a pulp. This is done by grinding the sample on a flat iron plate with an iron rubber, both of which have smooth surfaces, called a "buck-board and muller." This operation must be done very carefully to avoid losing any of the sample. . . . Use not less than a 60 mesh or preferably an 80 mesh sieve for pulp samples. The idea of pulverizing is not merely to disintegrate the ore so as to expose the minute particles to the action of the flux, but more especially to divide the free gold particles present so uniformly that in mixing the sample no one portion will contain coarser and heavier particles of gold than another.
Step 5. Verify the type of ore to know how much basic and/or reducing flux to add to the pulp:
1. Pure quartz - very glassy or vitreous - add Litharge and Reducing Agent so the slag will suspend the waste.
2. No quartz - Plastic and free from grit - add more silica or glass than usual to the flux.
3. A mixture of the two - add a little silica with the flux.
4. Sulfide ores - if large amounts are present, need to be roasted in a muffle and stirred frequently prior to fluxing.
Lesser amounts can be desulfurized by adding some large iron nails during melting and carefully removing before pouring into the pouring mold.

FLUX
Assay Litharge - Lead Oxide (PbO) - A Reddish, Orange or Yellow Powder milled to a fine and uniform particle size.
Reducing agent such as flour or argol - Potassium tartrate K2C4H4O6 - the potassium salt of tartaric acid.
NOTE: Don't use red lead oxide with silver as it tends to oxidize the silver and drive it into the cupel.
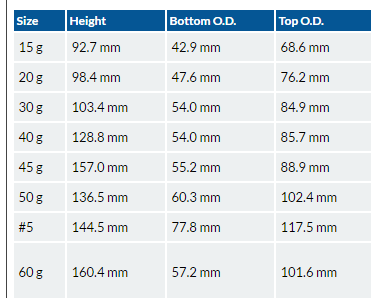
Assay Crucibles are available in sizes of 30, 40, 50, 55 and 65 grams.
A 30 gram crucible = 4 X 3 X 2 1/8" with a volume of 13.2 Cu.in.
A 40 gram crucible = 5 1/16 X 3 1/8 X 2 1/4" with a volume of 18.6 Cu.in.
A 50 gram crucible = 5 1/4 X 4 X 2.4" with a volume of 23 Cu.in.
The crucible commercially known as Battersea "B" or 20 gram size will be found convenient for use when 1 assay Ton of ore is taken; size "F" or 30 gram when 2 Assay Tons are used, together with the necessary amount of flux.
- J. Reginald Smith
- J. Reginald Smith
Never fill a crucible or scorifier more than three quarters full.
A 20 gram crucible holds 1A.T of pulp = 29.166 grams + 20 grams of litharge + reducing agent?
But what do I know? A question I still need to get answered. - 1dave
But what do I know? A question I still need to get answered. - 1dave
Answered by 911Metalurgest - Use a 30 gram crucible for 1 Assay Ton assays, and a 40 gram crucible for 2 ton assays.
Step 6. Weighing the sample

A Fire Assay begins by weighing out:
1 Assay Ton of pulp into each Fire Clay Crucible, then add flux of reducing agent and lead oxide. Stir.
For best noble metal collection from the pulp, Goal is to obtain around a 20 gram "Lead" Button.
Two or more agreeing assays assures accuracy of the reduced sample.
Use a larger crucible and 2 Assay Tons of pulp for lower grade ores to get more gold to weigh.
For High Grade Ores 1/10 A.T. may be desired.
An Assay Ton (A.T.) = 29.166 grams.
Definition of Assay Ton: A weights system created by combining aspects of Troy, Avoirdupois and Gram weights into a simple labor saving calculation unit. It was derived by reckoning in the following manner:
1 lb. av. = 7,000 troy grains.
2,000 lbs. = 1 Ton
2,000 X 7,000 = 14,000,000 Troy grains in 1 ton Av.
480 Troy grains = 1 oz Troy.
14,000,000/480 = 29,166 Troy oz. in 2,000 lbs. Av.
There are 29,166 milligrams in 1 assay ton (A.T.);
Therefore 2,000 lb. is to 1 A.T. as 1 oz. Troy is to 1 milligram.
So now using this system, if 1 A.T. of ore assays 1 milligram of gold or silver, the ton contains 1 ounce Troy.
Definition of Assay Ton: A weights system created by combining aspects of Troy, Avoirdupois and Gram weights into a simple labor saving calculation unit. It was derived by reckoning in the following manner:
1 lb. av. = 7,000 troy grains.
2,000 lbs. = 1 Ton
2,000 X 7,000 = 14,000,000 Troy grains in 1 ton Av.
480 Troy grains = 1 oz Troy.
14,000,000/480 = 29,166 Troy oz. in 2,000 lbs. Av.
There are 29,166 milligrams in 1 assay ton (A.T.);
Therefore 2,000 lb. is to 1 A.T. as 1 oz. Troy is to 1 milligram.
So now using this system, if 1 A.T. of ore assays 1 milligram of gold or silver, the ton contains 1 ounce Troy.
Step 7. Extracting, weighing, and calculating the values.
There are two methods of assaying:
the "Dry Assay" and the "Wet Assay."
the "Dry Assay" and the "Wet Assay."
Dry - AKA Fire Assay (assaying proper)- using heat, reducing fluxes. Includes cupellation and scorification.
Wet - AKA Chemical Assay (analysis)- includes volumetric, gravimetric, calorimetric, and electrolic.
Volumetric is the quickest and simplest as less manipulation is required. The sample may often be titrated without lengthy chemical separations.
The weight of ore taken for wet assay is much smaller than is required for Fire Assaying, 1/4 - 2 grams generally being sufficient. It is essential that the sample be finely crushed, especially if it is to be dissolved in acids, as particles of the mineral may be encased in the insoluble gangue. Minerals which are not decomposed by acids are fused with a flux which will render them soluble in water or acids.
The assay of gold and silver may be done by cupellation or by wet chemical volumetric methods. The cupellation method is subject to more errors than the wet assay. If accurate values are needed the chemical method must be applied.
DRY ASSAY

Dry Assay area
Fire Assay Cupels
Cupellation uses cupels traditionally made of bone ash (Ca5(OH)(PO4)3) because they have the ability to absorb metallic oxides. They are designed with a shallow bowl and heavy base for this purpose. Modern cupels have had Portland cement and/or Magnesite (MgCO3) added for strength as they must provide good durability as well as absorption of up to 70% of their weight in grams of lead.
Painting cupels with wet iron oxide to some extent prevents cutting by strong bases.
Magnesite cupels are more robust than their Bone Ash counterparts as they are not affected by temperature changes and are free from cracking and pitting. Magnesite cupels have a rapid rate of lead absorption, and the resulting dore beads are easily removed, minimizing losses.

Standard cupel sizes are #5, 6, 7A, 7AS, 8A, 8S, and 9.
A Fire assay begins with weighing a sample into a crucible and thoroughly blending it with a mixture of dry chemicals such as lead oxide and flour as a reducing agent.
The crucible is then placed into a 1000°C furnace.
The reducing agent reduces the lead oxide to lead. This forms thousands of small droplets of lead, which collect all the precious metals from the sample and sink to the bottom of the crucible.
Meanwhile, the non-precious metal contaminants are carried off into the slag.
The crucible is removed from the furnace, tapped to settle the metal, and poured into a mold.
Never take a scorification or cupellation from the furnace to finish at a future time. Complete the operation at once.
When cool, the slag is discarded and the "lead button" cleaned of any residual slag.
This entire process, therefore, selectively collects all the precious metals from the sample and alloys them with lead.
The next step is "cupellation". A heated cupel has the unique capability of absorbing metallic oxides.
The cupel is preheated to 950°C, then the lead button is placed into it.
Bring two of the cupels forward say within two inches of the front of the muffle. Place a lead button in each, using the cupel tongs, then shove the cupels back into the hotter part of the muffle. Close the muffle door for a few minutes. When the buttons have melted and cupellation has commenced, as shown by the fumes of litharge, open the door and arrange the dampers so that the fumes are drawn slowly off through the rear of the muffle.
Try to keep the temperature constant, increasing it only when the lead has almost disappeared.
The heat is too low when the fumes become heavy and dark, and a scum forms around the edge of the molten lead.
The heat is too high when the fumes of litharge rise rapidly to the top of the muffle and the lead appears to be boiling. The heat is just right when crystals of litharge form in the cupel.
Practice will enable you to fix upon the proper conditions.
Try to keep the temperature constant, increasing it only when the lead has almost disappeared.
The heat is too low when the fumes become heavy and dark, and a scum forms around the edge of the molten lead.
The heat is too high when the fumes of litharge rise rapidly to the top of the muffle and the lead appears to be boiling. The heat is just right when crystals of litharge form in the cupel.
Practice will enable you to fix upon the proper conditions.
The lead melts and the surface oxidizes.
The oxide is absorbed into the cupel, exposing more lead which oxidizes, etc.
As gold and silver do not oxidize until well above 1000°C, they continue to concentrate until the last of the lead is absorbed; leaving only a "dore bead" (containing only gold and silver) in the bottom of the cupel.
After cleaning, the beads' weight is recorded to get the total precious metal content of the sample.
The bead is then ''parted" by dissolving the silver from it with nitric acid which dissolves silver but does not affect gold.
After parting, the weight of gold is recorded.
Subtracting the gold weight from the dore weight provides the silver weight.
The problem with cupellation is that a small amount of gold and more silver is also absorbed into the cupel along with the lead, copper, and other volatile metal oxides.
Fire Assay Errors may be introduced during the following steps.
1. Sample Preparation include contamination from other samples, dilution by impurities, loss of fine particles, drying, and the degree of comminution ( breaking into small fragments) employed.
2. Fusion Process due to improper flux composition, temperature, and the size of the lead button obtained.
These errors cause the incomplete recovery of the precious metals in the lead button.
The metal lost is usually in the slag or crucible, and can be recovered by re-asssaying the slag in the original crucible.


WET ASSAYING

Wet assay area
forum.rocktumblinghobby.com/thread/62873/refining-silver
WET ASSAYING SILVER ORE: Is the same process usually used to Refine Silver.
From 1859 to 1891 the Comstock lode in Nevada produced $325,000,000. This lode is a belt of quartz in a contact vein between diorite and diabase.It is 10,000 feet long and several hundred feet wide.
The Anaconda mine in Butte was one of the largest producers of silver in the country. In 1896 its output was 5,000,000 ounces. The Anaconda was also the heaviest copper producer in the United States, its yield of copper being 125,350,693 pounds.
All silver ores are heavy, and many of them are sectile - may be cut with a knife.
Most silver ores are sulfides as silver easily enters into chemical combinations with sulfur.
In America galena is the principal source of silver. Next are the chlorides and oxides, and finally the silver that is parted from gold when it reaches the mint, as gold always contains some of that metal.
Silver is found in many different locations, and is associated with all sorts of minerals. It is never found in placer deposits, as it easily breaks up under the influence of water, air, etc. It is also found in igneous rocks in association with augite, hornblende and mica.
The commercial ores of silver are:
Argentite Ag2S - - - - - - .- 87.1 per cent silver
Cerargerite AgCl - - - - -. - 75.3 per cent silver
Stephanite 5Ag2SSb2S3 -- 68.5 per cent silver
Proustite 3Ag2SAs2S3 - - - 65.5 per cent silver
Pyrargyrite 3Ag2SSb2S3 - - 59.9 per cent silver
Silver miners have tested ore for silver by heating the ore and dipping it into water.
If silver is present some metal will come to the surface as a greasy scum.
Heated above 600°C silver tends to join with silica to form yellow crystals of silver silicate - Ag2O3Si. For this and other problems the cupellation assay of silver has been largely replaced by volumetric methods. Chief among these is the "Gay Lussac Assay", which was introduced in Paris in 1830 for testing silver bullion.
An exact weight of the bullion is dissolved in nitric acid, and very nearly all the silver is precipitated at once by the addition of a known volume of a standard solution of salt. When the precipitate has settled, the remaining silver is precipitated by the further addition of a small quantity of a more dilute solution of salt, the precipitate forming a white cloud in the supernatant liquid. The quantity of this silver is judged by the appearance of the white cloud.

Wet Assay of Silver Ore:
Begins as all assays - Collecting an honest sample, crushing, quartering, pulping, selecting final samples of 1/4 to 2 grams.
Now place the sample in a tube and dissolve the powdered ore in nitric acid.
The sulfur in the silver is replaced with Chlorine and turns from black to white.
Add a solution of common salt or hydrochloric acid to precipitate chloride of silver.
If chloride of lead and mercurous chloride are absent, then decant or filter the solution.
Dry and weigh the chloride of silver. Three fourths of the weight is nearly pure silver.
Collect and fuse the silver chloride to release the silver.
Weigh the metallic silver collected.
Once you have the weight of the silver extracted from the pulp, the following math will expand the numbers to the weight per ton of silver in the ore:
Weight of the sample in grams / 908000 grams per ton = weight of the sample in tons.
Or Weight of the sample in carats / 155.5 carats per troy ounce = wt. of the sample in ounces.
Next, to determine the ounces per ton, calculate "the Factor":
1/weight of the sample in tons = the Factor
Then the weight of the gold in troy ounces times the Factor = ounces of gold per ton of rock.
Finally, ounces times the spot price = the value.
Or Weight of the sample in carats / 155.5 carats per troy ounce = wt. of the sample in ounces.
Next, to determine the ounces per ton, calculate "the Factor":
1/weight of the sample in tons = the Factor
Then the weight of the gold in troy ounces times the Factor = ounces of gold per ton of rock.
Finally, ounces times the spot price = the value.

The web has numerous articles on assaying the other metals. For instance, Platinum is assayed by inductively coupled plasma optical emission spectrometry (ICP OES), which every rockhound should carry in his pocket.



 The charcoal crucible is made by lining an ordinary clay or Hessian crucible with a mixture of charcoal and molasses. The charcoal employed should be very fine, and only just enough molasses used to hold it together. The mixture is then packed into the crucible as tightly as possible, dried slowly, and bored out to any extent desirable. Sometimes water and gum are substituted for molasses. Fig. 9 represents three kinds of charcoal lined crucibles. Alumina crucibles for some operations are very satisfactory FIG. 9. when intense heat is required, but lime will answer as well. The choice of a crucible depends upon the nature of the substance to be treated in it, the temperature of the fire, and the time it is to remain exposed to the action of heat. If a charge be basic the crucible should be basic also, and vice versa. To test a crucible for fusibility, heat a piece of the crucible and see if the corners are rounded, or it is fused on the edges. For corosive action fuse litharge in the crucible. For permeability fill two crucibles with water and note the time required for it to run out; the one which holds the best being preferable. The action of sudden changes of temperature may be ascertained by heating suddenly, and cooling first in air and afterwards by plunging in cold water.
The charcoal crucible is made by lining an ordinary clay or Hessian crucible with a mixture of charcoal and molasses. The charcoal employed should be very fine, and only just enough molasses used to hold it together. The mixture is then packed into the crucible as tightly as possible, dried slowly, and bored out to any extent desirable. Sometimes water and gum are substituted for molasses. Fig. 9 represents three kinds of charcoal lined crucibles. Alumina crucibles for some operations are very satisfactory FIG. 9. when intense heat is required, but lime will answer as well. The choice of a crucible depends upon the nature of the substance to be treated in it, the temperature of the fire, and the time it is to remain exposed to the action of heat. If a charge be basic the crucible should be basic also, and vice versa. To test a crucible for fusibility, heat a piece of the crucible and see if the corners are rounded, or it is fused on the edges. For corosive action fuse litharge in the crucible. For permeability fill two crucibles with water and note the time required for it to run out; the one which holds the best being preferable. The action of sudden changes of temperature may be ascertained by heating suddenly, and cooling first in air and afterwards by plunging in cold water. ROASTING DISHES, (Fig. 10), and SCORIFIERS, (Fig. 11.) Both dishes and scorifiers are made of refractory clay. FIG. 11. They should FIG. 10. resist the action of litharge and not be too deep. Painting with water and oxide of iron prevents the cutting by strong bases, to some extent. Scorifiers may be bought or made, but as a rule it is better to buy them as they will stand transportation and require some care to make properly. A section of a good scorifier is uniform in character. It is close, and should show no flaws or cracks. (Fig. FIG. Ila. 11a.)
ROASTING DISHES, (Fig. 10), and SCORIFIERS, (Fig. 11.) Both dishes and scorifiers are made of refractory clay. FIG. 11. They should FIG. 10. resist the action of litharge and not be too deep. Painting with water and oxide of iron prevents the cutting by strong bases, to some extent. Scorifiers may be bought or made, but as a rule it is better to buy them as they will stand transportation and require some care to make properly. A section of a good scorifier is uniform in character. It is close, and should show no flaws or cracks. (Fig. FIG. Ila. 11a.)  CUPELS. These vessels are generally made of the ashes of burnt bones free from organic matter, ground and washed, horses or sheep bones are said to be the best. Cupels can be bought or made, but the latter is preferable when they have to be carried some distance. The prepared bone-ash can be obtained in bulk, and is mixed with just sufficient warm water to cause it to hold together without being moist. Sometimes in mixing the bone-ash a little wood ashes is added, or a spoonful of "pearl-ash," (carbonate of potash). Before adding it to the bone-ash it is dissolved in water. Too much bone-ash should not be mixed at once, as it dries quickly.
CUPELS. These vessels are generally made of the ashes of burnt bones free from organic matter, ground and washed, horses or sheep bones are said to be the best. Cupels can be bought or made, but the latter is preferable when they have to be carried some distance. The prepared bone-ash can be obtained in bulk, and is mixed with just sufficient warm water to cause it to hold together without being moist. Sometimes in mixing the bone-ash a little wood ashes is added, or a spoonful of "pearl-ash," (carbonate of potash). Before adding it to the bone-ash it is dissolved in water. Too much bone-ash should not be mixed at once, as it dries quickly.


 If much ore is to be pulverized, a grinding plate and rubber, (Buckboard and Muller)as shown in Fig. 17, will be a great convenience and save labor. The plate is a flat iron casting 18 X 24 inches, and 1 inch thick. The surface used being planed smooth. The rubber or grinder is a piece of cast iron, 4 X 6 inches square, 1 inches in the middle, by 7/8 of an inch thick at the ends; thus giving a slightly convex surface, which should be true on the board at all points. To conduct the operation place the left hand upon the rubber, throwing the weight of the body upon it, and then grasping the handle with the right hand, move the iron rubber back and forth, depressing the handle when pushing forward and raising it in drawing back. The operation is much more rapid than in the ordinary mortar and pestle style, and the manipulator after a little practice has complete control over the ore treated.
If much ore is to be pulverized, a grinding plate and rubber, (Buckboard and Muller)as shown in Fig. 17, will be a great convenience and save labor. The plate is a flat iron casting 18 X 24 inches, and 1 inch thick. The surface used being planed smooth. The rubber or grinder is a piece of cast iron, 4 X 6 inches square, 1 inches in the middle, by 7/8 of an inch thick at the ends; thus giving a slightly convex surface, which should be true on the board at all points. To conduct the operation place the left hand upon the rubber, throwing the weight of the body upon it, and then grasping the handle with the right hand, move the iron rubber back and forth, depressing the handle when pushing forward and raising it in drawing back. The operation is much more rapid than in the ordinary mortar and pestle style, and the manipulator after a little practice has complete control over the ore treated. A series of sieves, from twenty to one hundred mesh, will be useful for sifting ores and fluxes. The box sieve, (Fig. 18), is a simple arrangement, and consists of a round tin box with a sieve fitting into it as represented in the engraving. The sieve is a tin frame with any desired FIG. 18. mesh gauze soldered to it, and fits tightly in the box. The advantage gained by its use is that in sifting the pulverized ore there is no dust. The fine material being passed through the sieve is kept from flying around. The size most convenient is 8 inches in diameter, the box 2 inches deep, and the rim of the sieve 2 inches, fitting about 3/4 inch into the box.
A series of sieves, from twenty to one hundred mesh, will be useful for sifting ores and fluxes. The box sieve, (Fig. 18), is a simple arrangement, and consists of a round tin box with a sieve fitting into it as represented in the engraving. The sieve is a tin frame with any desired FIG. 18. mesh gauze soldered to it, and fits tightly in the box. The advantage gained by its use is that in sifting the pulverized ore there is no dust. The fine material being passed through the sieve is kept from flying around. The size most convenient is 8 inches in diameter, the box 2 inches deep, and the rim of the sieve 2 inches, fitting about 3/4 inch into the box. Cupel mould, (Fig. 19.). This consists of two parts, an iron ring and a steel pestle or driver, just fitting into the ring.
Cupel mould, (Fig. 19.). This consists of two parts, an iron ring and a steel pestle or driver, just fitting into the ring.  A mould for pouring the assay charge in scorification. (Fig. 20.) This should be of sheet iron or copper, and not too thin. It saves much time, and by employing it, the scorifiers can be used again.
A mould for pouring the assay charge in scorification. (Fig. 20.) This should be of sheet iron or copper, and not too thin. It saves much time, and by employing it, the scorifiers can be used again.  A tin sampler, shown in Fig. 21, will be found very useful. It consists of a series FIG. 21. of troughs arranged in a row and fastened together at equal distances by a tin strip soldered on their ends.
A tin sampler, shown in Fig. 21, will be found very useful. It consists of a series FIG. 21. of troughs arranged in a row and fastened together at equal distances by a tin strip soldered on their ends.
 One dozen parting flasks, (Fig. 24,) for gold bullion assay; also annealing cups for the same purpose. These are of clay and made thin. (Fig. 25.)
One dozen parting flasks, (Fig. 24,) for gold bullion assay; also annealing cups for the same purpose. These are of clay and made thin. (Fig. 25.)  Sulphuretted hydrogen is best prepared from powdered sulphide of iron (ferrous sulphide FeS) and dilute sulphuric acid. The gas being passed through a second bottle filled with water to wash it. Fig. 26 shows the apparatus in position for use The glass tubes are connected with small pieces of rubber tubing. The gas may be passed into a solution to be precipitated, or a water solution may be saturated and used at pleasure.
Sulphuretted hydrogen is best prepared from powdered sulphide of iron (ferrous sulphide FeS) and dilute sulphuric acid. The gas being passed through a second bottle filled with water to wash it. Fig. 26 shows the apparatus in position for use The glass tubes are connected with small pieces of rubber tubing. The gas may be passed into a solution to be precipitated, or a water solution may be saturated and used at pleasure.