|
|
Post by 1dave on Oct 13, 2022 11:03:56 GMT -5
en.wikipedia.org/wiki/Agate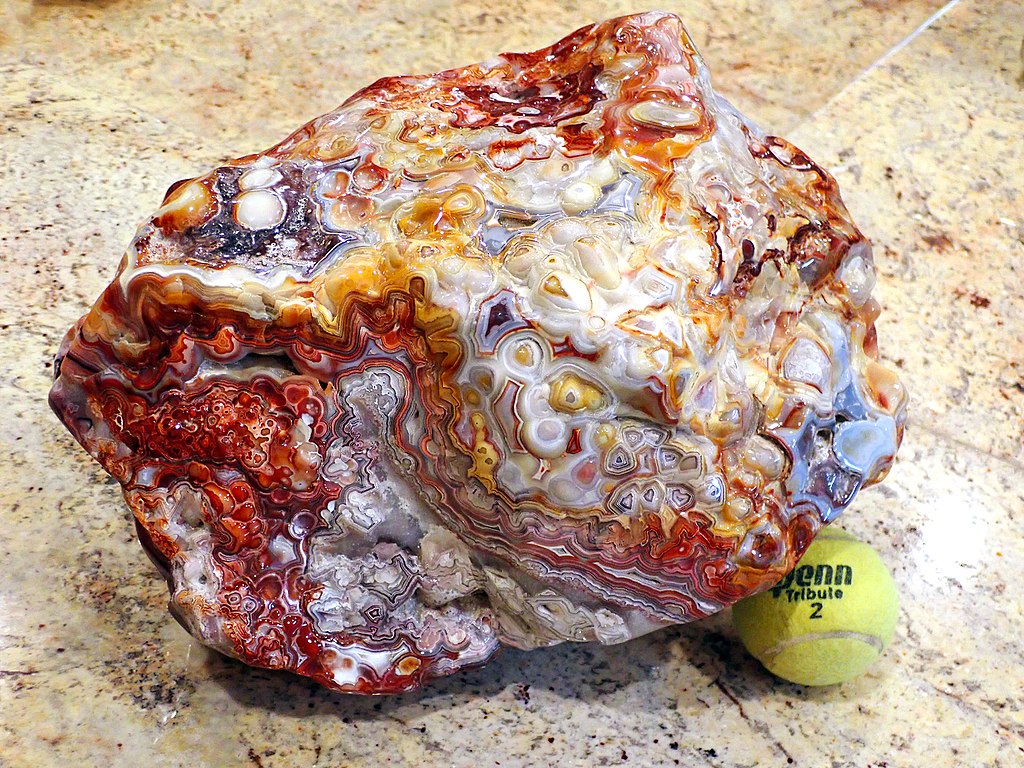 OLYMPUS DIGITAL CAMERA - Doxymo - Own work  Botswana agate - Pschemp 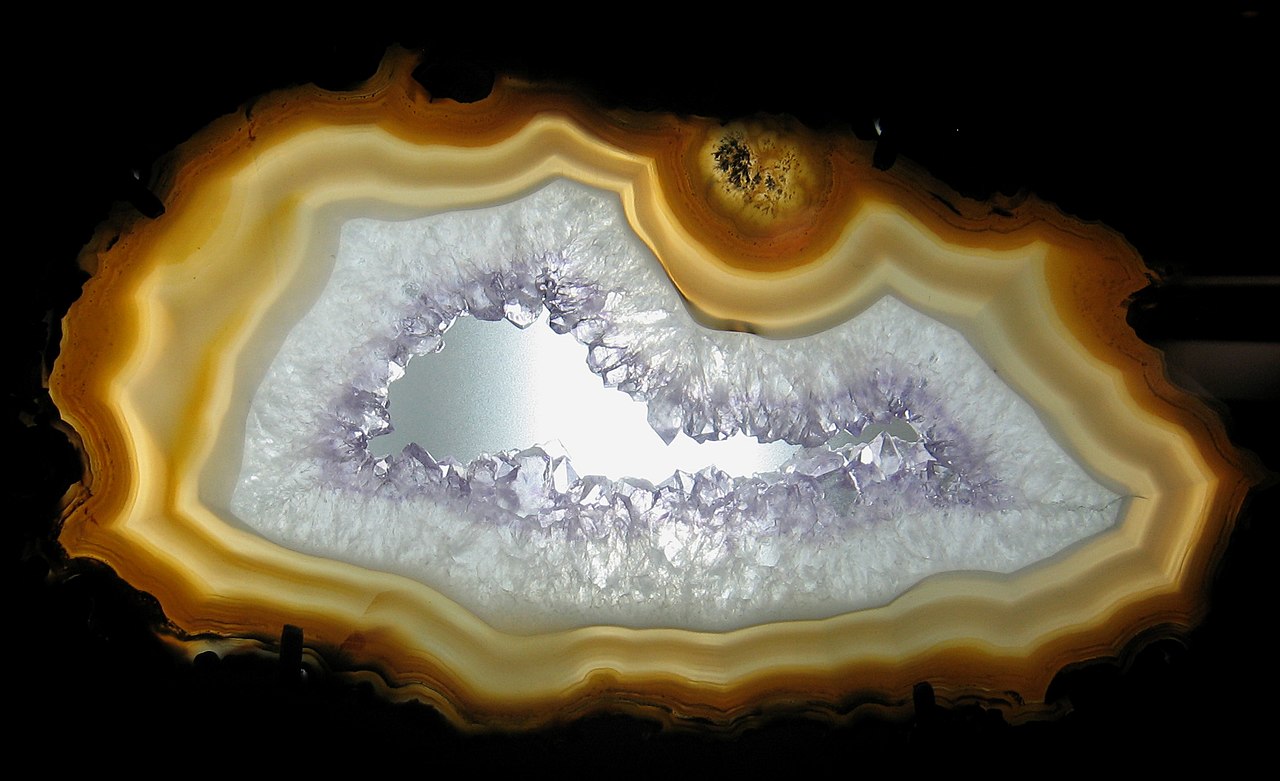 Hollow agate James St. John - Agate- & quartz-lined geode 5 I think the debris filled sphere was the original bottom, obviously a gas bubble in andesite.  Coyamito Agates From México - Agatesfm - Own work
How is agate formed?
|
|
|
|
Post by 1dave on Oct 13, 2022 11:35:58 GMT -5
Even the experts are perplexed! Agate in steam bubbles from basalt and andesite provide a lot of information. Add to that the agate that formed in steam ruptured crystobalite spheres known as lithophasae or thundereggs and the sequences become obvious, BUT what is the process? #1 Problem. transportation into the voids is obviously accomplished by groundwater. The least common dissolved mineral substances in groundwater are the silicates. The most common dissolved mineral substances in groundwater are the limestones and carbonates, specifically ions of sodium, calcium, magnesium, potassium; iron and manganese; chloride, bicarbonate, and sulfate. In water chemistry, these substances are called common constituents. 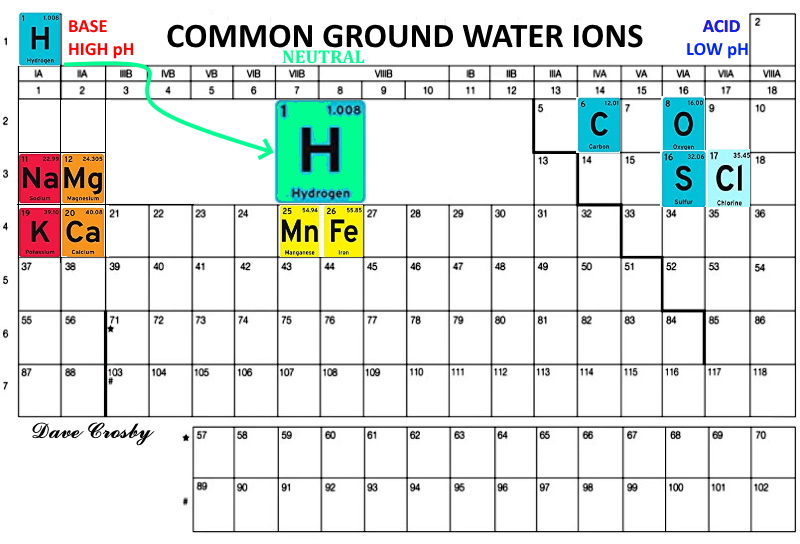 But Agate is mostly SiO2, the least soluble! 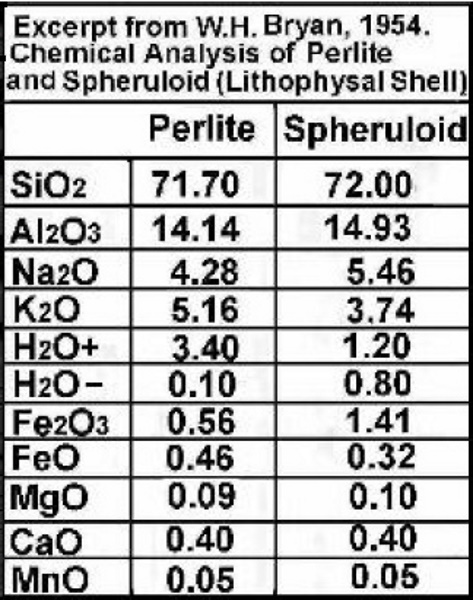 chemrxiv.org/engage/api-gateway/chemrxiv/assets/orp/resource/item/60c741aaf96a00fda52864a5/original/agate-analysis-by-raman-xrf-and-hyperspectral-imaging-spectroscopy-for-provenance-determination.pdf chemrxiv.org/engage/api-gateway/chemrxiv/assets/orp/resource/item/60c741aaf96a00fda52864a5/original/agate-analysis-by-raman-xrf-and-hyperspectral-imaging-spectroscopy-for-provenance-determination.pdf"Agate from the Idar-Oberstein is Permian in age (around 280 million years old). Agate from Rio Grande do Sul of Brazil generally formed during the Cretaceous (around 120 million years old)." Interesting, but is it accurate? That is when the gas bubbles formed, but not when they filled. In my opinion, agates are mostly chalcedony fibers with agate tips and scattered opal spheres. |
|
|
|
Post by oregon on Oct 13, 2022 17:37:39 GMT -5
The least common dissolved mineral substances in groundwater are the silicates. The most common dissolved mineral substances in groundwater are the limestones and carbonates, specifically ions of sodium, calcium, magnesium, potassium; iron and manganese; chloride, bicarbonate, and sulfate. I'm just going to offer up that Agate is mostly crystalline (micro that is) .
The Crystallization process is MUCH different than a precipitation event, and conditions for crystallization are different for different compounds.
Possibly all the limestone/carbonates/ionic compounds are much more soluble, so even if they did crystallize out of solution, over a long time scale, maybe conditions change enough where they redissolve until you get a compound that is much less soluble and unlikely to (re)dissolve.
The more I think about these sorta things, I realize that I really can't comprehend the time scales involved in these sorts of events, Ancient describes things a few thousand years ago, and these time scales are many orders of magnitude older :0
2c
|
|
|
|
Post by pauls on Oct 13, 2022 19:43:56 GMT -5
My observations in no particular order
I have a part of a geode that it is obvious the Agate was soft (a jell) when it formed, it is nicely banded but you can see where the Agate has become detached from the top of the gas bubble and has slumped down.
It is reasonably common in the Agates I collect from Agate Creek Queensland to find pseudomorphs of dogtooth Calcite in the geodes, not uncommon to find Aragonite /Agate pseudomorphs as well. I have a really nice smokey Quartz stalactite that has obviously formed around a Calcite stalactite that has been completely dissolved but Calcite crystal surfaces are still visible as hollows inside the smokey stalactite.
Colour doesn't appear to always be part of the silica jell as it is laid down in bands, colour seems to arrive later, You find blobs of colour in areas of geodes regardless of what the banding is doing.
|
|
|
|
Post by 1dave on Oct 13, 2022 21:55:58 GMT -5
Exactly oregon and paul! Calcium etc. crystalizes in our pipes when the pH is right, but huge caves are dissolved out of limestone when the pH is wrong. Steam bubbles up in a new basalt flow, cools down and becomes a hollow geode. Groundwater begins flowing thru it. Over time pH changes up and down. sediments settle. Crystals form. Settlements and crystals go. Time passes. Suddenly there is a major change and there is a LOT of silica gel in the water! |
|
|
|
AGATE
Oct 14, 2022 12:44:01 GMT -5
Post by 1dave on Oct 14, 2022 12:44:01 GMT -5
What makes that change? Thousands of years have passed and NOTHING HAS CHANGED. then EVERYTHING IS DIFFERENT! WHAT? HOW? The Answer = Shock waves! Land slides, earth quakes, impacts - Silica is brittle and shocks break the electron bonds! That is the long unknown difference. 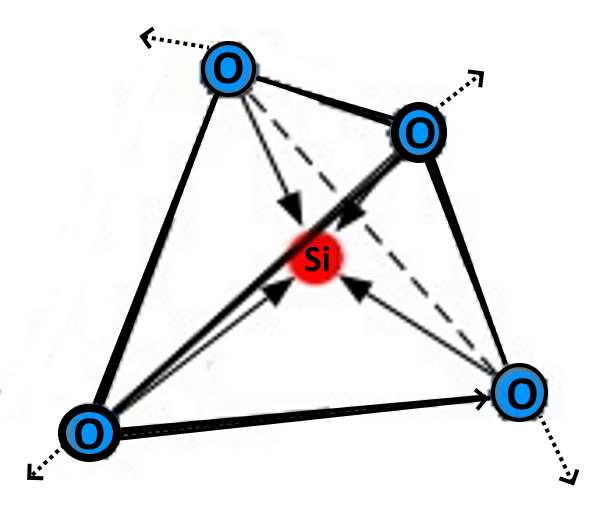 filling groundwater with ion chains of chalcedony, tridymite, crystobalite, etc. |
|