umm
1dave could you give a bit more detail? I know a bit about steel from my limited knifemaking experience, but I couldnt say what types of steel can shatter and what types cant.
Iron and most metals are soft and malleable. The more you pound it the brittler it becomes. Pound carbon into it and it becomes steel. Steel is much more brittle than soft iron.
When I was much younger I needed a chisel, but only had two rock picks. I thought I had the perfect solution and placed the pick end into the crack and pounded the hammer faces.
BAD MISTAKE! A needle of steel flew off one of the hammers, through my glove and into the base of my left glove. It took me two days to get back to town and my doctor. The hand was badly swollen and infected by then.
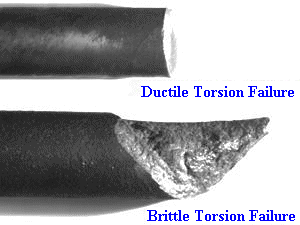
I still cringe every time I think about it.
en.wikipedia.org/wiki/Brittleness
Toughening
Graph comparing stress–strain curves for brittle and ductile materials
When a material has reached the limit of its strength, it usually has the option of either deformation or fracture. A naturally malleable metal can be made stronger by impeding the mechanisms of plastic deformation (reducing grain size, precipitation hardening, work hardening, etc.), but if this is taken to an extreme, fracture becomes the more likely outcome, and the material can become brittle. Improving material toughness is, therefore, a balancing act.
Naturally brittle materials, such as glass, are not difficult to toughen effectively. Most such techniques involve one of two mechanisms: to deflect or absorb the tip of a propagating crack or to create carefully controlled residual stresses so that cracks from certain predictable sources will be forced closed. The first principle is used in laminated glass where two sheets of glass are separated by an interlayer of polyvinyl butyral. The polyvinyl butyral, as a viscoelastic polymer, absorbs the growing crack. The second method is used in toughened glass and pre-stressed concrete. A demonstration of glass toughening is provided by Prince Rupert's Drop. Brittle polymers can be toughened by using metal particles to initiate crazes when a sample is stressed, a good example being high-impact polystyrene or HIPS. The least brittle structural ceramics are silicon carbide (mainly by virtue of its high strength) and transformation-toughened zirconia.
A different philosophy is used in composite materials, where brittle glass fibers, for example, are embedded in a ductile matrix such as polyester resin. When strained, cracks are formed at the glass–matrix interface, but so many are formed that much energy is absorbed and the material is thereby toughened. The same principle is used in creating metal matrix composites.
www.gowelding.com/met/carbon.htm Austenite This phase is only possible in carbon steel at high temperature. It has a Face Centre Cubic (F.C.C) atomic structure which can contain up to 2% carbon in solution.
Ferrite This phase has a Body Centre Cubic structure (B.C.C) which can hold very little carbon; typically 0.0001% at room temperature. It can exist as either: alpha or delta ferrite.
Carbon A very small interstitial atom that tends to fit into clusters of iron atoms. It strengthens steel and gives it the ability to harden by heat treatment. It also causes major problems for welding , particularly if it exceeds 0.25% as it creates a hard microstructure that is susceptible to hydrogen cracking. Carbon forms compounds with other elements called carbides. Iron Carbide, Chrome Carbide etc.
Cementite Unlike ferrite and austenite, cementite is a very hard intermetallic compound consisting of 6.7% carbon and the remainder iron, its chemical symbol is Fe3C. Cementite is very hard, but when mixed with soft ferrite layers its average hardness is reduced considerably. Slow cooling gives course perlite; soft easy to machine but poor toughness. Faster cooling gives very fine layers of ferrite and cementite; harder and tougher
Pearlite A mixture of alternate strips of ferrite and cementite in a single grain. The distance between the plates and their thickness is dependant on the cooling rate of the material; fast cooling creates thin plates that are close together and slow cooling creates a much coarser structure possessing less toughness. The name for this structure is derived from its mother of pearl appearance under a microscope. A fully pearlitic structure occurs at 0.8% Carbon. Further increases in carbon will create cementite at the grain boundaries, which will start to weaken the steel.
Cooling of a steel below 0.8% carbon When a steel solidifies it forms austenite. When the temperature falls below the A3 point, grains of ferrite start to form. As more grains of ferrite start to form the remaining austenite becomes richer in carbon. At about 723°C the remaining austenite, which now contains 0.8% carbon, changes to pearlite. The resulting structure is a mixture consisting of white grains of ferrite mixed with darker grains of pearlite. Heating is basically the same thing in reverse.
Martensite If steel is cooled rapidly from austenite, the F.C.C structure rapidly changes to B.C.C leaving insufficient time for the carbon to form pearlite. This results in a distorted structure that has the appearance of fine needles. There is no partial transformation associated with martensite, it either forms or it doesn’t. However, only the parts of a section that cool fast enough will form martensite; in a thick section it will only form to a certain depth, and if the shape is complex it may only form in small pockets. The hardness of martensite is solely dependant on carbon content, it is normally very high, unless the carbon content is exceptionally low.
Tempering The carbon trapped in the martensite transformation can be released by heating the steel below the A1 transformation temperature. This release of carbon from nucleated areas allows the structure to deform plastically and relive some of its internal stresses. This reduces hardness and increases toughness, but it also tends to reduce tensile strength. The degree of tempering is dependant on temperature and time; temperature having the greatest influence.
Annealing This term is often used to define a heat treatment process that produces some softening of the structure. True annealing involves heating the steel to austenite and holding for some time to create a stable structure. The steel is then cooled very slowly to room temperature. This produces a very soft structure, but also creates very large grains, which are seldom desirable because of poor toughness.
Normalising Returns the structure back to normal. The steel is heated until it just starts to form austenite; it is then cooled in air. This moderately rapid transformation creates relatively fine grains with uniform pearlite.
Welding If the temperature profile for a typical weld is plotted against the carbon equilibrium diagram, a wide variety of transformation and heat treatments will be observed.