Post by 1dave on Aug 9, 2023 7:34:49 GMT -5
Years ago I bought a piece of wood from Texas that was petrified with copper.


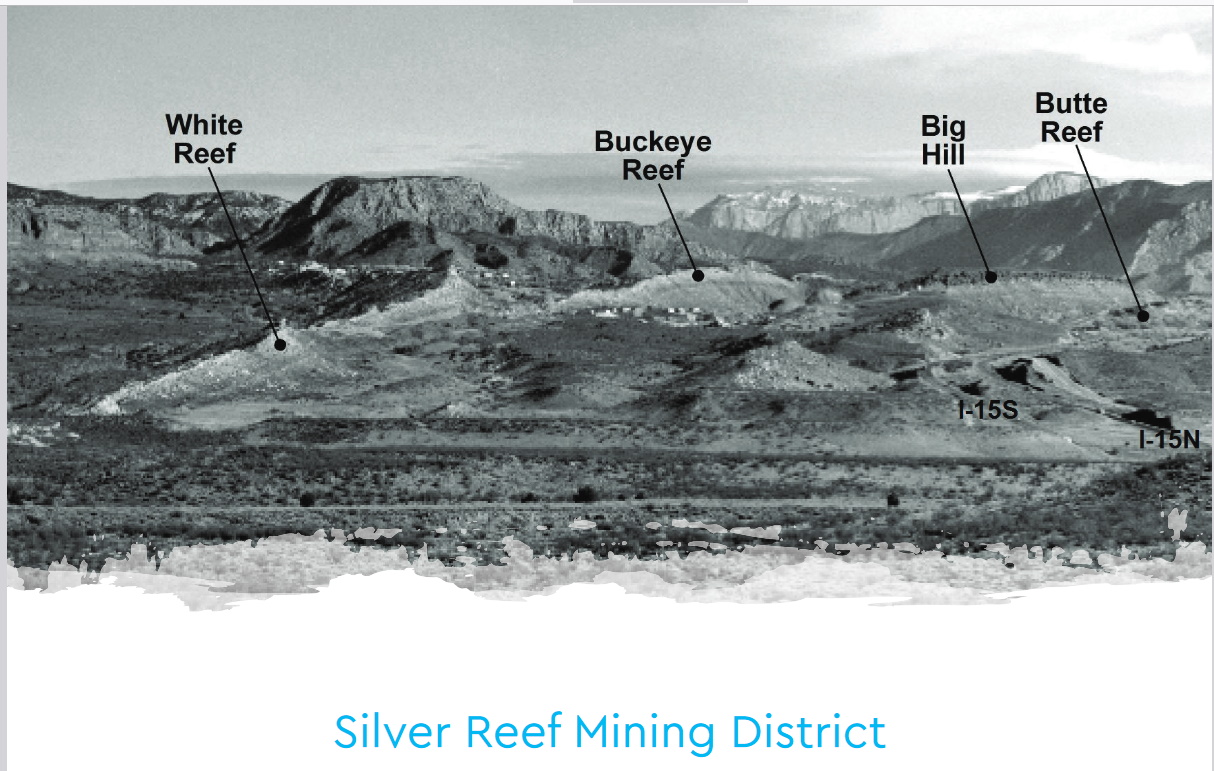
I live 40 miles from Silver Reef where the wood in a sandstone layer just above the famed Chinle Pet wood layer was replaced with Silver Chloride.

How was that possible?
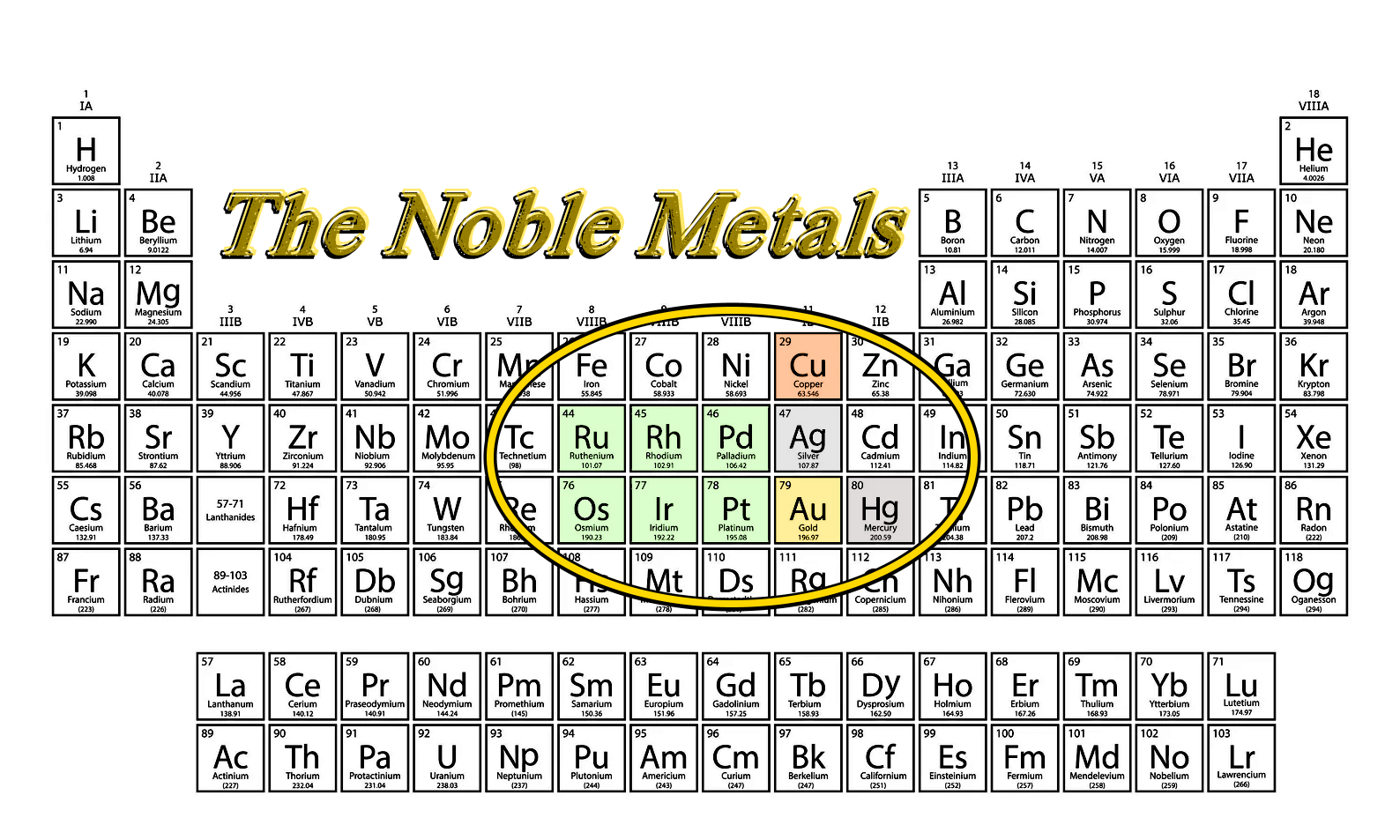
See where Copper, Silver, and GOLD are in the Periodic Chart?
Copper with water acts as a catalyst, converting carbon into carbon-dioxide, and takes it's place in the wood.
Silver is even better at that process. The latest I have found:
als.lbl.gov/a-new-path-to-carbon-dioxide-transformation/
May 17, 2019
SCIENTIFIC ACHIEVEMENT
Combining experiments at the Advanced Light Source (ALS) with quantum-mechanical calculations, scientists found dramatic differences in how carbon dioxide (CO2) reactions begin on silver as opposed to copper.
SIGNIFICANCE AND IMPACT
Both metals help transform CO2—a greenhouse gas—into more useful forms, and this new atomic-level data could help make the process more efficient.

Schematic drawings comparing molecular structures on copper and silver.
CO2 adsorption on copper (top left), silver (top right) and in the presence of H2O (bottom left and right). The depictions of CO2 on copper (from a previous study) show that linear and bent forms of CO2 (l-CO2 and b-CO2, respectively) are formed with CO2 adsorption alone and in the presence of H2O, aided by subsurface oxygen and surface H2O. In this study, the researchers found that CO2 on silver forms a completely new molecule (O=CO2δ−), similar in structure to carbonic acid (inset).
Rebalancing the carbon cycle
Fossil fuels are the lifeblood of modern societies, but their increased use releases carbon dioxide (CO2)—a greenhouse gas—into the atmosphere faster than plants can recycle it through photosynthesis. One way to address this is to reprocess CO2 into syngas, a feedstock for generating other useful chemicals. However, because CO2 is highly nonreactive, catalysts such as copper and silver are needed to facilitate the transformation (“reduction”) of CO2 into carbon monoxide (CO), a major component of syngas.
In searching for more efficient catalysts, scientists have computationally screened and lab-tested many materials. These approaches, however, have been based on preconceived notions about the relevant reaction mechanisms and have not produced dramatic successes. An atomic-level understanding of CO2 catalysis is essential to designing optimally performing materials.
Collaboration on copper
The Joint Center for Artificial Photosynthesis (JCAP) is a Department of Energy (DOE) Research Hub that aims to mimic photosynthesis—to produce renewable fuel using sunlight, and water, and CO2. In a previous collaboration, JCAP brought together theorists from Caltech and experimentalists from the ALS to study in detail what happens to CO2 at a copper surface.
In that study, the researchers found that subsurface oxygen in the copper dramatically boosts the early stages of CO2 catalysis. Bent CO2 (b-CO2) was shown to be a stable intermediate species in the presence of water. Such insights are essential to understanding, and eventually driving (using electrochemical processes), the reaction pathways to desired products.
A close-up of the silver lining
Now, a similar JCAP collaboration between the ALS and Caltech has expanded on the earlier work by investigating how CO2 and water molecules interact with a silver surface. They combined ambient-pressure x-ray photoelectron spectroscopy (APXPS) at ALS Beamline 9.3.2 with quantum-mechanical calculations to obtain a comprehensive understanding of the initial steps of CO2 adsorption and activation on silver.
Beamline 9.3.2 offers photons with a soft x-ray energy range that allows surface-sensitive probes, advancing investigations at solid/gas interfaces. The beamline endstation chamber allows excellent control of O2, CO2, and H2O gas pressures and reaction temperatures, important for accurately predicting the stable chemical species on the surface through simulations. A vacuum chamber attached to the analyzer allows in situ sample preparation and direct, pristine-sample measurement.
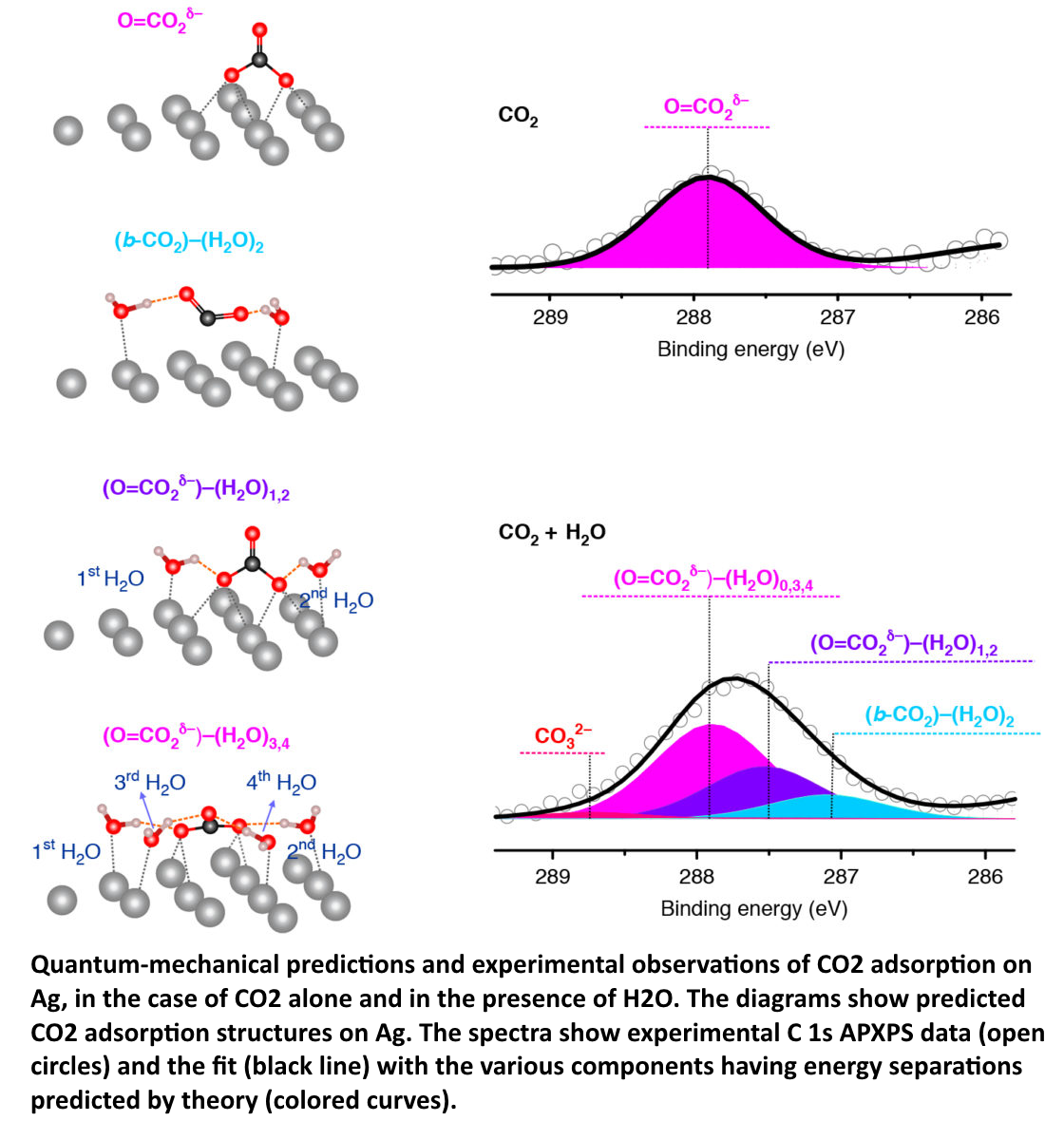
Schematic drawings of molecular structures on silver next to graphs of APXPS data.
Quantum-mechanical predictions and experimental observations of CO2 adsorption on Ag, in the case of CO2 alone and in the presence of H2O. The diagrams show predicted CO2 adsorption structures on Ag. The spectra show experimental C 1s APXPS data (open circles) and the fit (black line) with the various components having energy separations predicted by theory (colored curves).
A new reaction pathway
The results revealed a very different mechanism of CO2 activation on silver versus copper. A stable intermediate chemical species (O=CO2δ−) forms on silver when exposed to CO2 alone. In the presence of CO2 and water, the intermediate attaches up to four water molecules, and two water molecules stabilize b-CO2 as well. The new surface-chemisorbed species structurally resembles carbonic acid (H2CO3), but processes completely different electric properties. The (O=CO2δ−)–(H2O)n clusters on silver represent a significantly more favorable activation mechanism than b-CO2 on copper.
These unprecedented and surprising results—made possible using a synergistic and systematic experimental and computational approach—raise numerous questions about subsequent steps that will drive many new studies. Overall, the results lay a foundation for understanding the differences between catalysts, with the goal of controlling CO2 adsorption using additives or alloys and observing the reactions in process as they’re being electrochemically driven.
Photo of researchers in lab setting.
Ethan Crumlin, Yifan Ye, Jin Qian, and Junko Yano at Beamline 9.3.2.
Contact: Ethan Crumlin
Researchers: Y. Ye and E.J. Crumlin (ALS and Berkeley Lab); H. Yang, J. Qian, T. Cheng, H. Xiao, and W.A. Goddard III (California Institute of Technology); H. Su (ALS and University of Science and Technology of China); K.-J. Lee (ALS and Gwangju Institute of Science and Technology, South Korea); and J. Yano (Berkeley Lab).
Funding: U.S. Department of Energy, Office of Science, Basic Energy Sciences Program (DOE BES); China Scholarship Council; and National Science Foundation. Operation of the ALS is supported by DOE BES.
Publication: Y. Ye, H. Yang, J. Qian, H. Su, K.-J. Lee, T. Cheng, H. Xiao, J. Yano, W.A. Goddard III, and E.J. Crumlin, “Dramatic differences in carbon dioxide adsorption and initial steps of reduction between silver and copper,” Nat. Commun. 10, 1875 (2019), doi:10.1038/s41467-019-09846-y.
This work was also featured by Berkeley Lab as a Science Snapshot: “A ‘Silver Bullet’ for the Chemical Conversion of Carbon Dioxide.”
ALS SCIENCE HIGHLIGHT #396


I live 40 miles from Silver Reef where the wood in a sandstone layer just above the famed Chinle Pet wood layer was replaced with Silver Chloride.


How was that possible?
See where Copper, Silver, and GOLD are in the Periodic Chart?

Copper with water acts as a catalyst, converting carbon into carbon-dioxide, and takes it's place in the wood.
Silver is even better at that process. The latest I have found:
als.lbl.gov/a-new-path-to-carbon-dioxide-transformation/
A New Path to Carbon Dioxide Transformation
May 17, 2019
SCIENTIFIC ACHIEVEMENT
Combining experiments at the Advanced Light Source (ALS) with quantum-mechanical calculations, scientists found dramatic differences in how carbon dioxide (CO2) reactions begin on silver as opposed to copper.
SIGNIFICANCE AND IMPACT
Both metals help transform CO2—a greenhouse gas—into more useful forms, and this new atomic-level data could help make the process more efficient.

Schematic drawings comparing molecular structures on copper and silver.
CO2 adsorption on copper (top left), silver (top right) and in the presence of H2O (bottom left and right). The depictions of CO2 on copper (from a previous study) show that linear and bent forms of CO2 (l-CO2 and b-CO2, respectively) are formed with CO2 adsorption alone and in the presence of H2O, aided by subsurface oxygen and surface H2O. In this study, the researchers found that CO2 on silver forms a completely new molecule (O=CO2δ−), similar in structure to carbonic acid (inset).
Rebalancing the carbon cycle
Fossil fuels are the lifeblood of modern societies, but their increased use releases carbon dioxide (CO2)—a greenhouse gas—into the atmosphere faster than plants can recycle it through photosynthesis. One way to address this is to reprocess CO2 into syngas, a feedstock for generating other useful chemicals. However, because CO2 is highly nonreactive, catalysts such as copper and silver are needed to facilitate the transformation (“reduction”) of CO2 into carbon monoxide (CO), a major component of syngas.
In searching for more efficient catalysts, scientists have computationally screened and lab-tested many materials. These approaches, however, have been based on preconceived notions about the relevant reaction mechanisms and have not produced dramatic successes. An atomic-level understanding of CO2 catalysis is essential to designing optimally performing materials.
Collaboration on copper
The Joint Center for Artificial Photosynthesis (JCAP) is a Department of Energy (DOE) Research Hub that aims to mimic photosynthesis—to produce renewable fuel using sunlight, and water, and CO2. In a previous collaboration, JCAP brought together theorists from Caltech and experimentalists from the ALS to study in detail what happens to CO2 at a copper surface.
In that study, the researchers found that subsurface oxygen in the copper dramatically boosts the early stages of CO2 catalysis. Bent CO2 (b-CO2) was shown to be a stable intermediate species in the presence of water. Such insights are essential to understanding, and eventually driving (using electrochemical processes), the reaction pathways to desired products.
A close-up of the silver lining
Now, a similar JCAP collaboration between the ALS and Caltech has expanded on the earlier work by investigating how CO2 and water molecules interact with a silver surface. They combined ambient-pressure x-ray photoelectron spectroscopy (APXPS) at ALS Beamline 9.3.2 with quantum-mechanical calculations to obtain a comprehensive understanding of the initial steps of CO2 adsorption and activation on silver.
Beamline 9.3.2 offers photons with a soft x-ray energy range that allows surface-sensitive probes, advancing investigations at solid/gas interfaces. The beamline endstation chamber allows excellent control of O2, CO2, and H2O gas pressures and reaction temperatures, important for accurately predicting the stable chemical species on the surface through simulations. A vacuum chamber attached to the analyzer allows in situ sample preparation and direct, pristine-sample measurement.

Schematic drawings of molecular structures on silver next to graphs of APXPS data.
Quantum-mechanical predictions and experimental observations of CO2 adsorption on Ag, in the case of CO2 alone and in the presence of H2O. The diagrams show predicted CO2 adsorption structures on Ag. The spectra show experimental C 1s APXPS data (open circles) and the fit (black line) with the various components having energy separations predicted by theory (colored curves).
A new reaction pathway
The results revealed a very different mechanism of CO2 activation on silver versus copper. A stable intermediate chemical species (O=CO2δ−) forms on silver when exposed to CO2 alone. In the presence of CO2 and water, the intermediate attaches up to four water molecules, and two water molecules stabilize b-CO2 as well. The new surface-chemisorbed species structurally resembles carbonic acid (H2CO3), but processes completely different electric properties. The (O=CO2δ−)–(H2O)n clusters on silver represent a significantly more favorable activation mechanism than b-CO2 on copper.
These unprecedented and surprising results—made possible using a synergistic and systematic experimental and computational approach—raise numerous questions about subsequent steps that will drive many new studies. Overall, the results lay a foundation for understanding the differences between catalysts, with the goal of controlling CO2 adsorption using additives or alloys and observing the reactions in process as they’re being electrochemically driven.
Photo of researchers in lab setting.
Ethan Crumlin, Yifan Ye, Jin Qian, and Junko Yano at Beamline 9.3.2.
Contact: Ethan Crumlin
Researchers: Y. Ye and E.J. Crumlin (ALS and Berkeley Lab); H. Yang, J. Qian, T. Cheng, H. Xiao, and W.A. Goddard III (California Institute of Technology); H. Su (ALS and University of Science and Technology of China); K.-J. Lee (ALS and Gwangju Institute of Science and Technology, South Korea); and J. Yano (Berkeley Lab).
Funding: U.S. Department of Energy, Office of Science, Basic Energy Sciences Program (DOE BES); China Scholarship Council; and National Science Foundation. Operation of the ALS is supported by DOE BES.
Publication: Y. Ye, H. Yang, J. Qian, H. Su, K.-J. Lee, T. Cheng, H. Xiao, J. Yano, W.A. Goddard III, and E.J. Crumlin, “Dramatic differences in carbon dioxide adsorption and initial steps of reduction between silver and copper,” Nat. Commun. 10, 1875 (2019), doi:10.1038/s41467-019-09846-y.
This work was also featured by Berkeley Lab as a Science Snapshot: “A ‘Silver Bullet’ for the Chemical Conversion of Carbon Dioxide.”
ALS SCIENCE HIGHLIGHT #396











