Post by 1dave on Feb 15, 2018 10:37:37 GMT -5
I've always heard of gold being found in quartz veins, but in the Liberty mining district it is found in calcite!
Here Nick is explaining the gold is only found where basalt feeder dikes pass through shale beds.

Soak the calcite in acid and the gold crystals are released.

Gold is also found below where it has been deposited by weathering.

If you want to watch the presentation:

goldrefiningforum.com/~goldrefi/phpBB3/viewtopic.php?t=21271
Re: Precipitating carbonates
Quote
Postby Gratilla » September 30th, 2014, 6:16 pm
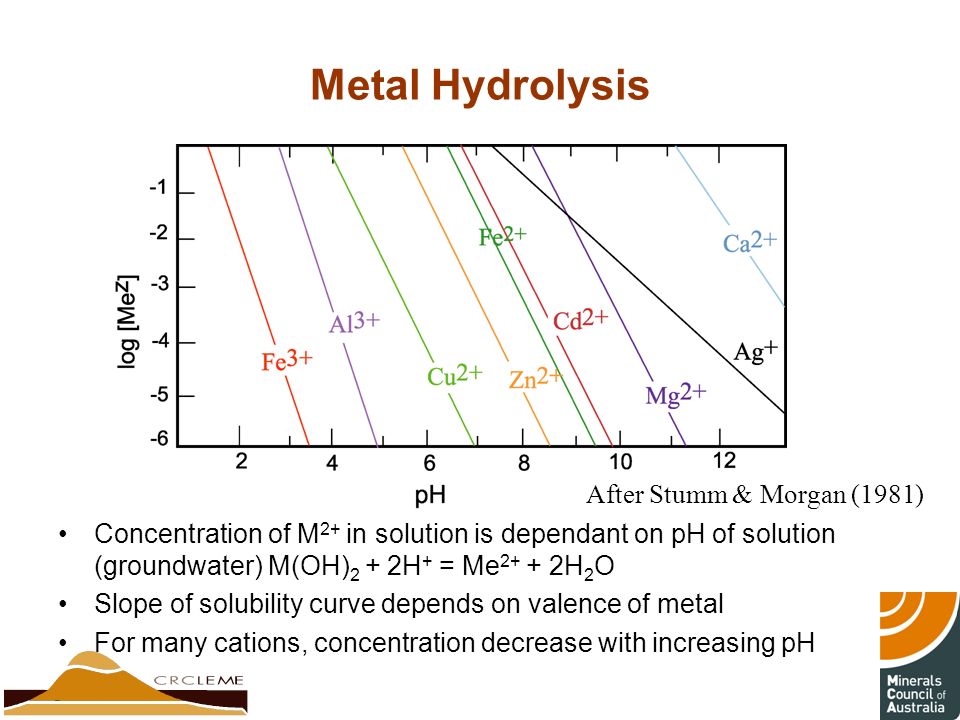
Yes. Do a google on "metal [carbonate/hydroxide] solubility curves" and choose "picture" (ie not the default "web" links) and you'll be shown a slew of graphs.
You'll note (as you mentioned) that pH/solubility behaviour varies with cation, anion, etc. Some compound solubilities will decrease with increasing pH, some decrease with increasing pH, many decrease then increase and some are not much affected. Note that the solubility scale is usually logarithmic (pH is also, of course, logarithmic), so there can be a very significant solubility change with a relatively small change in pH. AND solubility can also change fairly significantly with temperature. So careful choice of pH and temp can produce quite effective separations.
In fact, the mining industry uses calcium oxide/hydroxide and calcium carbonate on acid-side leaches for exactly this purpose, dropping (for example) As and Fe salts at around pH 2.5 and other hydroxides/carbonates (selectively) as they gradually increase pH.
www.miningeducation.com/2011/11/types-of-gold-veins-in-ining.html
Here Nick is explaining the gold is only found where basalt feeder dikes pass through shale beds.

Soak the calcite in acid and the gold crystals are released.

Gold is also found below where it has been deposited by weathering.

If you want to watch the presentation:

goldrefiningforum.com/~goldrefi/phpBB3/viewtopic.php?t=21271
Re: Precipitating carbonates
Quote
Postby Gratilla » September 30th, 2014, 6:16 pm
Yes. Do a google on "metal [carbonate/hydroxide] solubility curves" and choose "picture" (ie not the default "web" links) and you'll be shown a slew of graphs.
You'll note (as you mentioned) that pH/solubility behaviour varies with cation, anion, etc. Some compound solubilities will decrease with increasing pH, some decrease with increasing pH, many decrease then increase and some are not much affected. Note that the solubility scale is usually logarithmic (pH is also, of course, logarithmic), so there can be a very significant solubility change with a relatively small change in pH. AND solubility can also change fairly significantly with temperature. So careful choice of pH and temp can produce quite effective separations.
In fact, the mining industry uses calcium oxide/hydroxide and calcium carbonate on acid-side leaches for exactly this purpose, dropping (for example) As and Fe salts at around pH 2.5 and other hydroxides/carbonates (selectively) as they gradually increase pH.

Re: Precipitating carbonates
Quote
Postby Geo » October 1st, 2014, 11:36 am
Thank you everyone. While processing CPU's, it occurred to me that there may be a way to clean the solution of bothersome metals first and leave the precious metals in solution. Just removing the iron would be an advantage so when I came across this and it was talking about the same thing but added lead and zinc. I'm so worried about arsenic from CPU's but apparently, it precipitates out as well. The more I learn about this, the more amazed I am at what can be done.
Quote
Postby Geo » October 1st, 2014, 11:36 am
Thank you everyone. While processing CPU's, it occurred to me that there may be a way to clean the solution of bothersome metals first and leave the precious metals in solution. Just removing the iron would be an advantage so when I came across this and it was talking about the same thing but added lead and zinc. I'm so worried about arsenic from CPU's but apparently, it precipitates out as well. The more I learn about this, the more amazed I am at what can be done.
www.miningeducation.com/2011/11/types-of-gold-veins-in-ining.html











