Post by 1dave on Jun 14, 2018 7:17:28 GMT -5
The Bare Essentials of Matter:

en.wikipedia.org/wiki/Quark
In 1963, when I assigned the name "quark" to the fundamental constituents of the nucleon, I had the sound first, without the spelling, which could have been "kwork". Then, in one of my occasional perusals of Finnegans Wake, by James Joyce, I came across the word "quark" in the phrase "Three quarks for Muster Mark". Since "quark" (meaning, for one thing, the cry of the gull) was clearly intended to rhyme with "Mark", as well as "bark" and other such words, I had to find an excuse to pronounce it as "kwork". But the book represents the dream of a publican named Humphrey Chimpden Earwicker. Words in the text are typically drawn from several sources at once, like the "portmanteau" words in Through the Looking-Glass. From time to time, phrases occur in the book that are partially determined by calls for drinks at the bar. I argued, therefore, that perhaps one of the multiple sources of the cry "Three quarks for Muster Mark" might be "Three quarts for Mister Mark", in which case the pronunciation "kwork" would not be totally unjustified. In any case, the number three fitted perfectly the way quarks occur in nature. - Murray Gell-Mann
George Zweig preferred the name ace for the particle he had theorized, but Gell-Mann's terminology came to prominence once the quark model had been commonly accepted.
George Zweig preferred the name ace for the particle he had theorized, but Gell-Mann's terminology came to prominence once the quark model had been commonly accepted.
A quark (/kwɔːrk, kwɑːrk/) is a type of elementary particle and a fundamental constituent of matter. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei.[1] Due to a phenomenon known as color confinement, quarks are never directly observed or found in isolation; they can be found only within hadrons, which exist as either baryons (which include protons and neutrons) or as mesons.[2][3] For this reason, much of what is known about quarks has been drawn from observations of the hadrons themselves.
Quarks have various intrinsic properties, including electric charge, mass, color charge, and spin. They are the only elementary particles in the Standard Model of particle physics to experience all four fundamental interactions (electromagnetism, gravitation, strong interaction, and weak interaction)—also known as fundamental forces. And quarks are the only known particles whose electric charges are not integer multiples of their elementary charges.
There are six types (or flavors) of quarks: up, down, charm, strange, top, and bottom (see infobox above, "Generations of matter"). [4] The up/down generation (also called "family") of quarks has the lowest masses of all the generations (families). The heavier quarks rapidly change into up/down quarks through a process known as particle decay, which is the persistent transformation from a higher mass state to a lower mass state. Thus, up/down quarks are generally stable and the most common in the universe, whereas the charm/strange and top/bottom families of quarks can only be produced in high energy collisions (such as those involving cosmic rays and particle accelerators). For every quark flavor there is a corresponding type of antiparticle known as an antiquark, which differs from the quark only in that some of its properties, such as charge have equal magnitude but opposite sign.
The quark model was independently proposed by physicists Murray Gell-Mann and George Zweig in 1964.[5] Quarks were introduced as parts of an ordering scheme for hadrons, and there was little evidence for their physical existence until deep inelastic scattering experiments at the Stanford Linear Accelerator Center in 1968.[6][7] Accelerator experiments have provided evidence for all six flavors. The top quark was the last to be discovered at Fermilab in 1995.[5]
The Standard Model is the theoretical framework describing all the currently known elementary particles. This model contains six flavors of quarks (q), named up (u), down (d), charm (c), strange (s), top (t), and bottom (b).[4]
Antiparticles of quarks are called antiquarks, and are denoted by a bar over the symbol for the corresponding quark, such as u for an up antiquark. As with antimatter in general, antiquarks have the same mass, mean lifetime, and spin as their respective quarks, but the electric charge and other charges have the opposite sign.[8]
Quarks are spin-1⁄2 particles, implying that they are fermions according to the spin–statistics theorem. They are subject to the Pauli exclusion principle, which states that no two identical fermions can simultaneously occupy the same quantum state. This is in contrast to bosons (particles with integer spin), any number of which can be in the same state.[9] Unlike leptons, quarks possess color charge, which causes them to engage in the strong interaction. The resulting attraction between different quarks causes the formation of composite particles known as hadrons (see "Strong interaction and color charge" below).
The quarks that determine the quantum numbers of hadrons are called valence quarks; apart from these, any hadron may contain an indefinite number of virtual (or sea) quarks, antiquarks, and gluons, which do not influence its quantum numbers.[10] There are two families of hadrons: baryons, with three valence quarks, and mesons, with a valence quark and an antiquark.[11] The most common baryons are the proton and the neutron, the building blocks of the atomic nucleus.[12] A great number of hadrons are known (see list of baryons and list of mesons), most of them differentiated by their quark content and the properties these constituent quarks confer. The existence of "exotic" hadrons with more valence quarks, such as tetraquarks and pentaquarks, were conjectured from the beginnings of the quark model[13] but not discovered until the early 21st century.[14][15][16][17]
Elementary fermions are grouped into three generations, each comprising two leptons and two quarks. The first generation includes up and down quarks, the second strange and charm quarks, and the third bottom and top quarks. All searches for a fourth generation of quarks and other elementary fermions have failed,[18][19] and there is strong indirect evidence that no more than three generations exist.[nb 1][20][21][22] Particles in higher generations generally have greater mass and less stability, causing them to decay into lower-generation particles by means of weak interactions. Only first-generation (up and down) quarks occur commonly in nature. Heavier quarks can only be created in high-energy collisions (such as in those involving cosmic rays), and decay quickly; however, they are thought to have been present during the first fractions of a second after the Big Bang, when the universe was in an extremely hot and dense phase (the quark epoch). Studies of heavier quarks are conducted in artificially created conditions, such as in particle accelerators.[23]
Having electric charge, mass, color charge, and flavor, quarks are the only known elementary particles that engage in all four fundamental interactions of contemporary physics: electromagnetism, gravitation, strong interaction, and weak interaction.[12] Gravitation is too weak to be relevant to individual particle interactions except at extremes of energy (Planck energy) and distance scales (Planck distance). However, since no successful quantum theory of gravity exists, gravitation is not described by the Standard Model.
See the table of properties above for a more complete overview of the six quark flavors' properties.
Proton
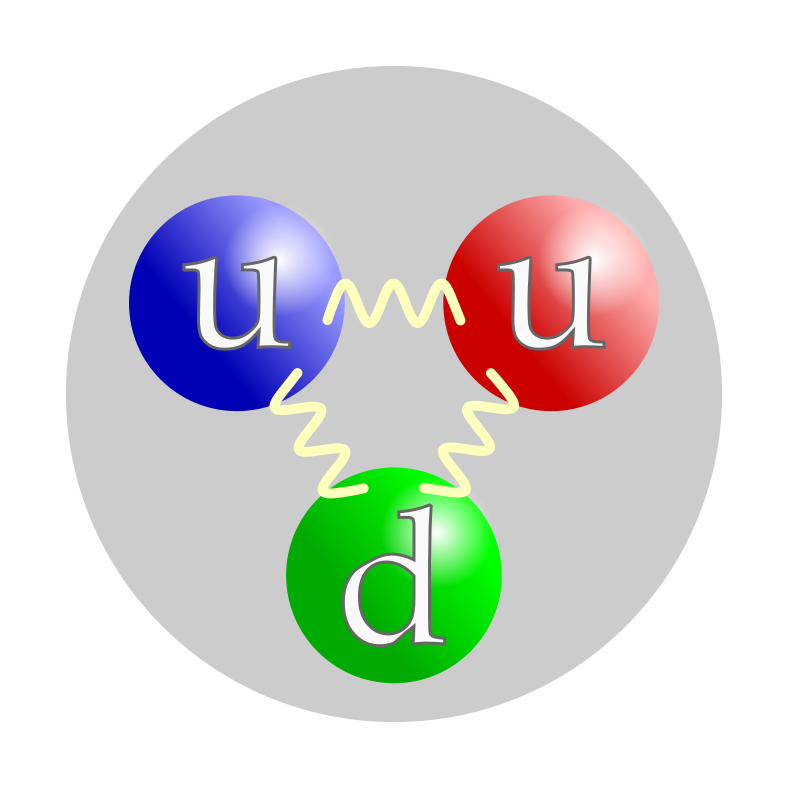

A proton is a subatomic particle, symbol p or p+, with a positive electric charge of +1e elementary charge and mass slightly less than that of a neutron. Protons and neutrons, each with masses of approximately one atomic mass unit, are collectively referred to as "nucleons".
One or more protons are present in the nucleus of every atom; they are a necessary part of the nucleus. The number of protons in the nucleus is the defining property of an element, and is referred to as the atomic number (represented by the symbol Z). Since each element has a unique number of protons, each element has its own unique atomic number.
The word proton is Greek for "first", and this name was given to the hydrogen nucleus by Ernest Rutherford in 1920. In previous years, Rutherford had discovered that the hydrogen nucleus (known to be the lightest nucleus) could be extracted from the nuclei of nitrogen by atomic collisions. Protons were therefore a candidate to be a fundamental particle, and hence a building block of nitrogen and all other heavier atomic nuclei.
In the modern Standard Model of particle physics, protons are hadrons, and like neutrons, the other nucleon (particles present in atomic nuclei), are composed of three quarks. Although protons were originally considered fundamental or elementary particles, they are now known to be composed of three valence quarks: two up quarks and one down quark. The rest masses of quarks contribute only about 1% of a proton's mass, however.[3] The remainder of a proton's mass is due to quantum chromodynamics binding energy, which includes the kinetic energy of the quarks and the energy of the gluon fields that bind the quarks together. Because protons are not fundamental particles, they possess a physical size, though not a definite one; the root mean square charge radius of a proton is about 0.84–0.87 fm or 0.84×10−15 to 0.87×10−15 m.[4][5]
At sufficiently low temperatures, free protons will bind to electrons. However, the character of such bound protons does not change, and they remain protons. A fast proton moving through matter will slow by interactions with electrons and nuclei, until it is captured by the electron cloud of an atom. The result is a protonated atom, which is a chemical compound of hydrogen. In vacuum, when free electrons are present, a sufficiently slow proton may pick up a single free electron, becoming a neutral hydrogen atom, which is chemically a free radical. Such "free hydrogen atoms" tend to react chemically with many other types of atoms at sufficiently low energies. When free hydrogen atoms react with each other, they form neutral hydrogen molecules (H2), which are the most common molecular component of molecular clouds in interstellar space.
One or more protons are present in the nucleus of every atom; they are a necessary part of the nucleus. The number of protons in the nucleus is the defining property of an element, and is referred to as the atomic number (represented by the symbol Z). Since each element has a unique number of protons, each element has its own unique atomic number.
The word proton is Greek for "first", and this name was given to the hydrogen nucleus by Ernest Rutherford in 1920. In previous years, Rutherford had discovered that the hydrogen nucleus (known to be the lightest nucleus) could be extracted from the nuclei of nitrogen by atomic collisions. Protons were therefore a candidate to be a fundamental particle, and hence a building block of nitrogen and all other heavier atomic nuclei.
In the modern Standard Model of particle physics, protons are hadrons, and like neutrons, the other nucleon (particles present in atomic nuclei), are composed of three quarks. Although protons were originally considered fundamental or elementary particles, they are now known to be composed of three valence quarks: two up quarks and one down quark. The rest masses of quarks contribute only about 1% of a proton's mass, however.[3] The remainder of a proton's mass is due to quantum chromodynamics binding energy, which includes the kinetic energy of the quarks and the energy of the gluon fields that bind the quarks together. Because protons are not fundamental particles, they possess a physical size, though not a definite one; the root mean square charge radius of a proton is about 0.84–0.87 fm or 0.84×10−15 to 0.87×10−15 m.[4][5]
At sufficiently low temperatures, free protons will bind to electrons. However, the character of such bound protons does not change, and they remain protons. A fast proton moving through matter will slow by interactions with electrons and nuclei, until it is captured by the electron cloud of an atom. The result is a protonated atom, which is a chemical compound of hydrogen. In vacuum, when free electrons are present, a sufficiently slow proton may pick up a single free electron, becoming a neutral hydrogen atom, which is chemically a free radical. Such "free hydrogen atoms" tend to react chemically with many other types of atoms at sufficiently low energies. When free hydrogen atoms react with each other, they form neutral hydrogen molecules (H2), which are the most common molecular component of molecular clouds in interstellar space.
Electron

The electron is a subatomic particle, symbol e− or β− , with a negative elementary electric charge.[8] Electrons belong to the first generation of the lepton particle family,[9] and are generally thought to be elementary particles because they have no known components or substructure.[1] The electron has a mass that is approximately 1/1836 that of the proton.[10] Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value, expressed in units of the reduced Planck constant, ħ. As it is a fermion, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle.[9] Like all elementary particles, electrons exhibit properties of both particles and waves: they can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer De Broglie wavelength for a given energy.
Electrons play an essential role in numerous physical phenomena, such as electricity, magnetism, chemistry and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions.[11] Since an electron has charge, it has a surrounding electric field, and if that electron is moving relative to an observer it will generate a magnetic field. Electromagnetic fields produced from other sources (not those self-produced) will affect the motion of an electron according to the Lorentz force law. Electrons radiate or absorb energy in the form of photons when they are accelerated. Laboratory instruments are capable of trapping individual electrons as well as electron plasma by the use of electromagnetic fields. Special telescopes can detect electron plasma in outer space. Electrons are involved in many applications such as electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.
Interactions involving electrons with other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between the positive protons within atomic nuclei and the negative electrons without, allows the composition of the two known as atoms. Ionization or differences in the proportions of negative electrons versus positive nuclei changes the binding energy of an atomic system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding.[12] In 1838, British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms.[3] Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897.[5][13][14] Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons can be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles can be totally annihilated, producing gamma ray photons.
Electrons play an essential role in numerous physical phenomena, such as electricity, magnetism, chemistry and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions.[11] Since an electron has charge, it has a surrounding electric field, and if that electron is moving relative to an observer it will generate a magnetic field. Electromagnetic fields produced from other sources (not those self-produced) will affect the motion of an electron according to the Lorentz force law. Electrons radiate or absorb energy in the form of photons when they are accelerated. Laboratory instruments are capable of trapping individual electrons as well as electron plasma by the use of electromagnetic fields. Special telescopes can detect electron plasma in outer space. Electrons are involved in many applications such as electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.
Interactions involving electrons with other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between the positive protons within atomic nuclei and the negative electrons without, allows the composition of the two known as atoms. Ionization or differences in the proportions of negative electrons versus positive nuclei changes the binding energy of an atomic system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding.[12] In 1838, British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms.[3] Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897.[5][13][14] Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons can be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles can be totally annihilated, producing gamma ray photons.
Neutron

The proton has a positive electric charge of +1e and mass slightly less than that of a neutron.
The electron has a negative electric charge of -1e and mass that is approximately 1/1836 that of the proton.
The neutron is a subatomic particle, symbol n or n0, with no net electric charge and a mass slightly larger than that of a proton.
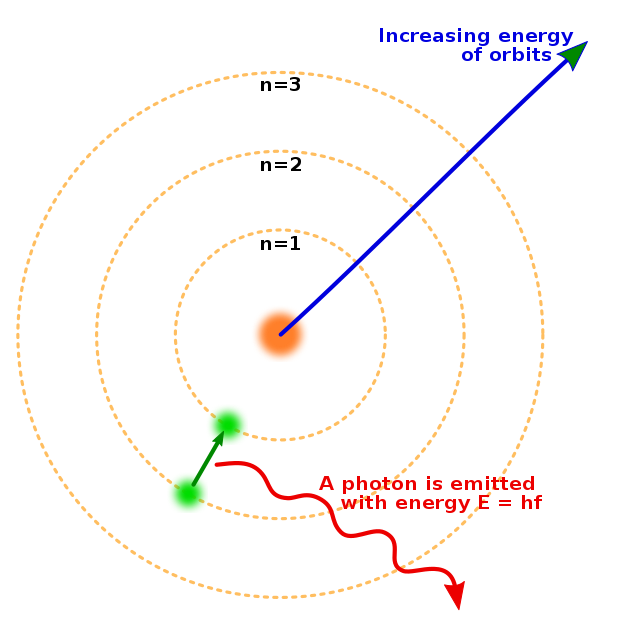
Isn't a neutron simply a scrunched together hydrogen atom with an extremely tight electron orbit?


The neutron is a subatomic particle, symbol n or n0, with no net electric charge and a mass slightly larger than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons.[5] Their properties and interactions are described by nuclear physics.
The chemical and nuclear properties of the nucleus are determined by the number of protons, called the atomic number, and the number of neutrons, called the neutron number. The atomic mass number is the total number of nucleons. For example, carbon has atomic number 6, and its abundant carbon-12 isotope has 6 neutrons, whereas its rare carbon-13 isotope has 7 neutrons. Some elements occur in nature with only one stable isotope, such as fluorine. Other elements occur with many stable isotopes, such as tin with ten stable isotopes.
Within the nucleus, protons and neutrons are bound together through the nuclear force, and neutrons are required for the stability of nuclei. Neutrons are produced copiously in nuclear fission and fusion. They are a primary contributor to the nucleosynthesis of chemical elements within stars through fission, fusion, and neutron capture processes.
The neutron is essential to the production of nuclear power. In the decade after the neutron was discovered by James Chadwick in 1932,[6] neutrons were used to induce many different types of nuclear transmutations. With the discovery of nuclear fission in 1938,[7] it was quickly realized that, if a fission event produced neutrons, each of these neutrons might cause further fission events, etc., in a cascade known as a nuclear chain reaction.[8] These events and findings led to the first self-sustaining nuclear reactor (Chicago Pile-1, 1942) and the first nuclear weapon (Trinity, 1945).
Free neutrons, or individual neutrons free of the nucleus, are a form of ionizing radiation and, as such, are a biological hazard, depending upon dose.[8] A small natural "neutron background" flux of free neutrons exists on Earth, caused by cosmic ray showers, and by the natural radioactivity of spontaneously fissionable elements in the Earth's crust.[9] Dedicated neutron sources like neutron generators, research reactors and spallation sources produce free neutrons for use in irradiation and in neutron scattering experiments.
The chemical and nuclear properties of the nucleus are determined by the number of protons, called the atomic number, and the number of neutrons, called the neutron number. The atomic mass number is the total number of nucleons. For example, carbon has atomic number 6, and its abundant carbon-12 isotope has 6 neutrons, whereas its rare carbon-13 isotope has 7 neutrons. Some elements occur in nature with only one stable isotope, such as fluorine. Other elements occur with many stable isotopes, such as tin with ten stable isotopes.
Within the nucleus, protons and neutrons are bound together through the nuclear force, and neutrons are required for the stability of nuclei. Neutrons are produced copiously in nuclear fission and fusion. They are a primary contributor to the nucleosynthesis of chemical elements within stars through fission, fusion, and neutron capture processes.
The neutron is essential to the production of nuclear power. In the decade after the neutron was discovered by James Chadwick in 1932,[6] neutrons were used to induce many different types of nuclear transmutations. With the discovery of nuclear fission in 1938,[7] it was quickly realized that, if a fission event produced neutrons, each of these neutrons might cause further fission events, etc., in a cascade known as a nuclear chain reaction.[8] These events and findings led to the first self-sustaining nuclear reactor (Chicago Pile-1, 1942) and the first nuclear weapon (Trinity, 1945).
Free neutrons, or individual neutrons free of the nucleus, are a form of ionizing radiation and, as such, are a biological hazard, depending upon dose.[8] A small natural "neutron background" flux of free neutrons exists on Earth, caused by cosmic ray showers, and by the natural radioactivity of spontaneously fissionable elements in the Earth's crust.[9] Dedicated neutron sources like neutron generators, research reactors and spallation sources produce free neutrons for use in irradiation and in neutron scattering experiments.
The electron has a negative electric charge of -1e and mass that is approximately 1/1836 that of the proton.
The neutron is a subatomic particle, symbol n or n0, with no net electric charge and a mass slightly larger than that of a proton.
Isn't a neutron simply a scrunched together hydrogen atom with an extremely tight electron orbit?
Hydrogen


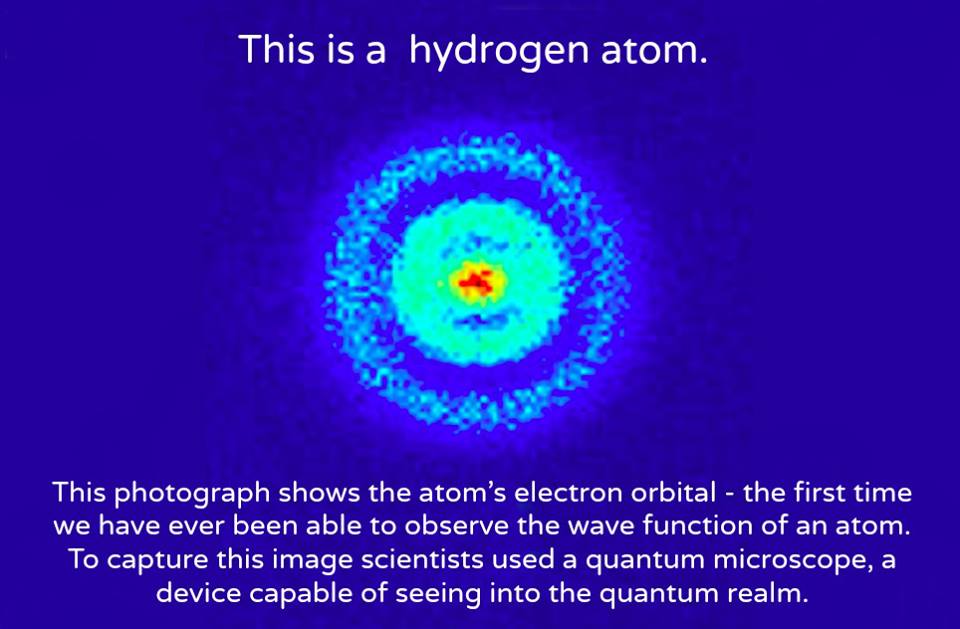
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the baryonic mass of the universe.[1]
In everyday life on Earth, isolated hydrogen atoms (called "atomic hydrogen") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. "Atomic hydrogen" and "hydrogen atom" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).
Attempts to develop a theoretical understanding of the hydrogen atom have been important to the history of quantum mechanics.
In everyday life on Earth, isolated hydrogen atoms (called "atomic hydrogen") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. "Atomic hydrogen" and "hydrogen atom" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).
Attempts to develop a theoretical understanding of the hydrogen atom have been important to the history of quantum mechanics.











