|
|
Post by 1dave on Mar 31, 2020 0:55:38 GMT -5
I noticed a statement that "Tripole magnets do not exist." Well, of course they do!
They are all around us. Examine a water molecule.  Tripole Water Molecule The electron clouds are thicker in the bonding regions of molecules, leaving the molecules slightly negative on the oxygen end, and slightly positive on the TWO hydrogen ends - making the molecules TRIPOLES! "Hydrogen bonding" connects the water molecules together in weak chains, but double strong at the surface creating surface tension with a repelling face.  Magnetic Water Surface Of course the same is true for SiO2 and similar molecules.  |
|
|
|
Post by 1dave on Mar 31, 2020 14:57:01 GMT -5
Tripole magnets were mentioned in 1976, but nobody payed attention. 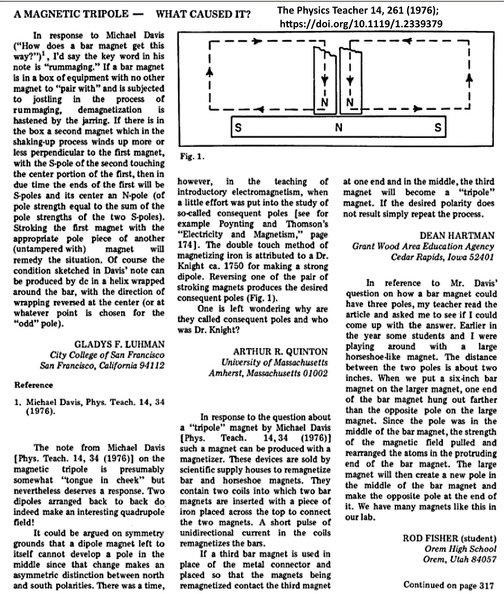 |
|
|
|
Post by 1dave on Apr 1, 2020 13:50:23 GMT -5
Why are water molecule bonds "bent"? Because of the spare electron pressure.  |
|