H2O Molecular Geometry, Lewis Structure, Shape and Bond Angles
Janice Powell October 26, 2017
geometryofmolecules.com/h2o-lewis-structure-shape-and-bond-angles/0 18,208 2 minutes read - H2O Molecular Geometry
As you may know, I have already explained about the molecular shape of CO2, SO2, SO3, SF4, and XeF4. Today, it’s time to learn about the molecular form of H2O. We all know that it is the formula of water and also one of the most well-known, common chemical recipes. In this article, I am going to give useful and easy to understand information of H2O molecular geometry and H2O Lewis structure to my super young followers. So stay tuned and find all the answers to your confusions regarding the chemical composition of water.
Before we start, here is some interesting fact. We all know the chemical formula of water that is H2O. But, do you know there is another name of water which is quite unfamiliar? Water can also be called as the not-so-known chemical name of Dihydrogen Monoxide Hoax (DHMO.
Contents
What is the electronic geometry of H2O?
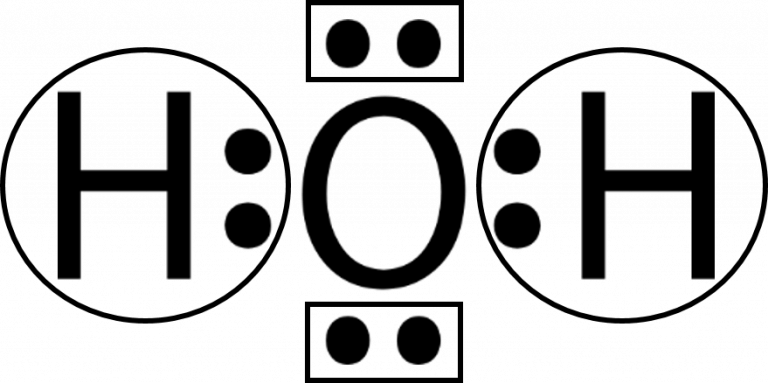
In order to determine the molecular geometry for H2O, observe the Lewis structure of the same. There is an atom of oxygen in the center and two atoms of hydrogen around the central atom. There are also two pairs of electrons around the oxygen, which you can see at the Lewis structure.
So, according to Valence Shell Electron Pair Repulsion (VSEPR) Theory, all of these will spread out as far as possible, which will end up to giving us the shape of H2O.
H2O Lewis Structure
H2O Lewis Structure
Molecule Shape of H2O
There are three dimensions of H2O, which can help us to visualize the shape of this chemical formula of water. Look at this picture. Here, the pink atom is the Oxygen, and the white molecules are Hydrogen. There is also one lone pair of electron above all of these.
While observing this image, we can find out that this is not a straight molecule. In reality, it contains a bent shape which can be called as the molecular geometry bent.
Molecule Shape of H2O
There is one another method, which can help us to find out the shape of such compositions. There is an option of AXN method.
axn
‘A’ stands for the central atom, which is none other than oxygen in H2O. It contains one atom. ‘X’ informs us about the number of atoms attached to the central atom. As we have two atoms of hydrogen, X is going to be ‘2.’ Lastly, the ‘N’ stands for the notion that is the number of nonbonding electron pairs. Here, there is no pair of nonbonding electrons.
ax2n
Here, we have two pairs of nonbonding electron pairs;
H2O electron geometry
So, N will be zero. Thus, the outcome is – AX2N2
Now, go to the table to find out the shape and the bond angles of various molecular geometries. If you want, you can also memorize the stuff as it is not at all complicated and advantageous. Here is the table you should keep in mind for better and fast results.
There is also an easy way available. Look it up on this table.
Formula Shape Bond Angles
AX2 Linear 180
AX3 Trigonal planar 120
AX4 Tetrahedral 109.5
AX5 Triangular Bipyramidal 120, 90
AX6 Octahedral 90
AX2N Bent 120
AX2N2 Bent 109.5
AX3N Trigonal pyramidal 109.5
Once finding out, you will see that the AX2N2 has a ‘Bent Molecular Geometry.’ H2O, which is a three atom molecule, comes with the angular shape.
H2O Bond Angles
Looking at the table, when we go from AX2, AX3 and all the way down to AX2N2, we will find out that the bond angle is going to be 109.5 degrees.
So, using both the Valence Shell Electron Pair Repulsion (VSEPR) Theory and the table where we look at the AXN, we can quickly know about the molecular geometry for water.
I hope you have found this article of the H2O electron geometry easy to understand and useful. The geometry of molecules is actually not a complicated subject to know about if you pay proper attention to the formulas and fundamentals. It is essential to be clear in some basic concepts, and there you go! You can crack any method, find out their shapes, and able to understand the bond angles. Keep trying, keep learning, and if possible, keep explaining to others!