rrod
having dreams about rocks

Member since December 2020
Posts: 72
|
Post by rrod on Dec 5, 2021 16:21:27 GMT -5
Dropbox or one of the "Drives" (iCloud, Google, OneDrive). Not entirely sure what you mean by that as a route to posting here. They should all give you a means to share a link that will allow someone to download the file. You could then post that link here. If you mean you want the PDF to show up like a picture, I'm not sure if the board supports that natively. You could always convert the PDF to a picture format and then post that picture. |
|
|
|
Post by Bob on Dec 5, 2021 16:35:29 GMT -5
[on Dec 6 I deleted the image in this post and replaced it with a much better one visible on that day below]
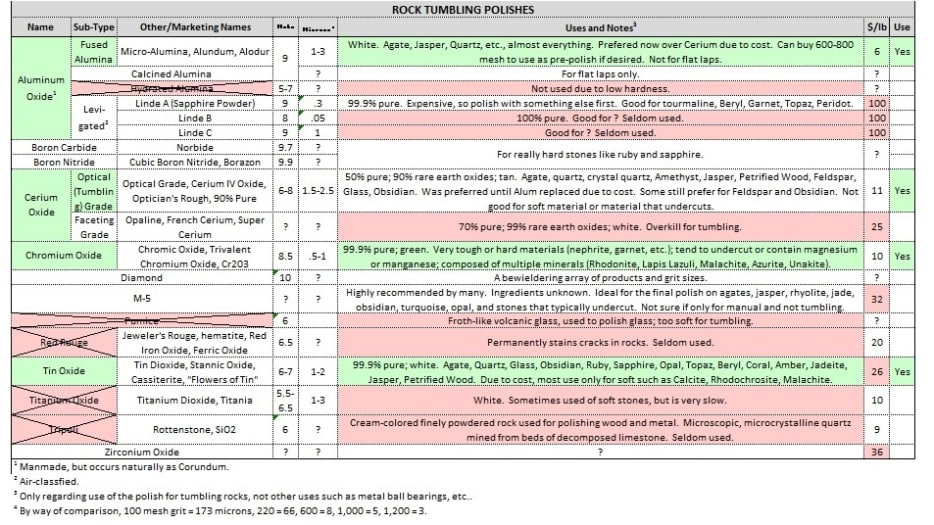
That is what a survey of literature I did in 2015 found. Based upon it, I guess I'm surprised tit ox/diox is shipped as you say with those new tumblers. Perhaps I was led astray in publications. I know image is lousy. 5th col is Microns link to footnote 4.
|
|
|
|
Post by Bob on Dec 5, 2021 16:36:48 GMT -5
Not entirely sure what you mean by that as a route to posting here. They should all give you a means to share a link that will allow someone to download the file. You could then post that link here. If you mean you want the PDF to show up like a picture, I'm not sure if the board supports that natively. You could always convert the PDF to a picture format and then post that picture. Thanks. The link didn't work from Google Drive. But I converted to a jpg though I think lost some clarity in image. |
|
|
|
Post by Mel on Dec 5, 2021 16:43:55 GMT -5
This is so incredibly fascinating to me! I use titanium dioxide and I'm finding I can get a polish in 7-10 days on what I tumble - typically the basics; aventurine, quartz, amethyst, jaspers, agates. I can't wait to see your nephrite results, any results really!. I love the look of nephrite/jadeite, but have only seen highly polished pieces retail, never from a hobbyist. I think when I read this when you posted, I mistakenly read tin ox instead of what you actually wrote. Maybe I could take one set of rocks from this test, knock the polish off with 1,000 SC, then do a run in titanium ? (did you really mean diox not ox?) too. If it's decently available and not exorbitant. Someone mentioned trying diamond dust too which I know nothing about. Yep, titanium dioxide. It's what I used when I first was polishing rocks (also used it in soap making!), but haven't used it since I ran out because I read about the carcinogenic properties.... HOWEVER, like an idiot, I didn't pay attention or I would have realized 2 things: 1) I was reading literature from California where literally everything has that warning/gives you cancer and 2) That that was due to incredibly long exposure to high amounts of it, more than I would ever possibly come in contact with! Now I can't find it anywhere in large amounts, but I also haven't really been looking... |
|
|
|
Post by Bob on Dec 6, 2021 11:40:02 GMT -5
 I just realized another way to do it is to print it out, then take a photo of the print, which I'm trying right now and will see if better than that pdf to jpg conversion. |
|
|
|
Post by Bob on Dec 6, 2021 11:43:16 GMT -5
That looks better so I'm going to delete that prior post image.
The 5th column is Microns and footnote 4. This was my attempt to understand the polishes. In my spreadsheet work, green means good and red means bad.
When I get this polish comparison test done, I will probably add the results to this table for future reference. Like the rocks themselves, sorting out the various trade names of the polishes was time consuming.
I remember now being puzzled about the M-5 polish. If anyone knows about that I would enjoying finding out.
|
|
|
|
Post by Bob on Dec 9, 2021 10:22:33 GMT -5
Decided that I should not use that tin oxide I ordered from new vendor on Amazon, when all others are from known quality vendor KN. So am ordering more from KN. I feel strongly that for this test to mean anything I must have fewest variables possible.
|
|
|
|
Post by Bob on Dec 12, 2021 23:15:56 GMT -5
|
|
|
|
Post by Bob on Dec 12, 2021 23:20:37 GMT -5
From my memory of the other 3 polishes, this is almost but not quite as shiny overall as Tin. The nephrite is the same as the others. Calcite almost gone, but dull not shiny. Hematite shiny but with lousy edges.
Have gone ahead and started week 2 in Alum.
|
|
|
|
Post by Bob on Dec 12, 2021 23:24:57 GMT -5
Week 2 done with Cerium and Chromium. But I'm not going to comment on them until all 4 polishes reach that point. Then I will compare each rock.
Also will not burnish until after that as I believe this comparison test should be before any burnishing.
The new Tin hasn't arrived yet so week 2 in that hasn't started yet.
|
|
|
|
Post by Bob on Dec 13, 2021 10:13:05 GMT -5
I was not expecting this test to go as well as it has in one way. There was a worry that the obsidian, glass, lapis, feldspars, and other touchy materials would be damaged by being polished with tough, common materials like jaspers and agates and especially with a piece of heavy hematite in the batch. Although I have not inspected things with a magnifying glass, so far this doesn't seem to have happened.
When I was accumulating material for this test, I was actually worried that it might not fit into a 6lb barrel, so I tried to go smaller with everything. However, there was so much space left over filled with plastic beads, that I could have gone much larger with all materials. These loads are 75% or more plastic beads. That I suppose could have it's own effect--good or bad I don't know. In a typical polish run for me I have 25% or less plastic beads, and sometimes none at all if there is a good mix of sizes in the material.
|
|
|
|
Post by Bob on Dec 17, 2021 14:38:39 GMT -5
I would like some input from those with rock photography knowledge as well as anyone that is enjoying this test.
As an example of the photos I hope to take--or perhaps thought maybe I should take--I would put all 4 pieces of lapis, one from each of the 4 polishes, side by side so you can see them and see if you agree or disagree as to why I think one polish did best for that material.
What would be the ideal way to do this? A flat black surface but with a lamp attempting to get a shiny reflection spot on each rock in the photo?
Another thing is I could have the polish label showing on something that sticks up behind the rock so that all who see it see which polish before they even look at how the rocks compare. But, another way would be that I don't do that on purpose, but label each rock with the polish it came out of (T/A/Chr/Cer) with a small felt tip Sharpie on the bottom of the rock not visible in the photo. I could see if you agree that this or that rock is better than the others, and then after reveal which polish it was. In other words, I would not put the rocks in that T/A/Chr/Cer order from left to right otherwise everyone would know which is which even w/o seeing any labelling.
Or, if that kind of participation in this process doesn't sound interesting to others, I could also just write the results for each rock type and not even bother with photos.
As much work as has been put into this, it would gratify me to wrap this up in a way that benefits others and is fun for others. Given when the additional tin oxide is expected to arrive from KN, and that alum ox will be completely done in a day or two, I anticipate being able to start sharing the results between Christmas and New Years.
|
|
|
|
Post by Bob on Dec 25, 2021 22:16:22 GMT -5
2nd week in tin ox just started...last step.
|
|
jawsjr
starting to shine!

Member since June 2019
Posts: 45
|
Post by jawsjr on Jan 11, 2022 14:37:18 GMT -5
Is there an update to this?
|
|
|
|
Post by Bob on Jan 11, 2022 17:49:15 GMT -5
jawsjr, yes, unfortunately the first problem has occurred. When I opened the 2nd week done in tin ox, it was apparent something wasn't right. The plastic beads together with the rocks were sort of mostly pasted around the circumference--not dry--but also not watery as usual. They didn't fall inward after removing the lid either. It was as if insufficient water had been added to the load, but I don't think that's possible because I did them all the same. Also, I've never had a failed polish run like this in 7+ years of doing them.
So I added about a cup of water, ran it for 30 more minutes, and then it looked more or less right. However, many of the rocks are damaged. Whether they were damaged by what I just described, or from a broken rock I found, I don't know. A piece of spiderwoman jasper had broken apart and maybe it was the culprit. I also looked on the tumbler base to see if the barrel had leaked, but could not be certain because I hadn't cleaned it up in a while and it was pretty dirty. However, I would think dried white slurry would be pretty easy to spot and I didn't see any. So this is most perplexing. I can actually remember the water lifting the plastic beads like I always see.
So, the bad news is I've got to start all over with the tin oxide run, going back to zero with it, two full weeks. About half the rocks were undamaged, especially the hard jaspers and agates, so they are ready to roll again. But, with some types of rocks, I didn't have enough in ready-to-polish inventory. So for those, I'm having to put some polished ones back in 1,000 to take off the polish and get them ready.
It looks though in a week I will be ready to restart it except for one type, the exotica jasper. I have no other pieces and it was pretty damaged and one week in 1,000 will not be enough to fix it. I'll just have to remove it from the comparison.
With me leaving to Quartzite soon, this mishap has postponed the final comparison probably into February.
I've been thinking about how to compare and have decided to take photos on a low tripod with my Olympus Tough Camera. I just bought a circular flash light diffuser that goes around the lens. Hopefully this will enable anyone in this thread to feel like they are with me when doing the final comparisons.
|
|
|
|
Post by Bob on Jan 11, 2022 17:52:21 GMT -5
 Here's what they look like. You might not be able to see much damage, but there is a lot. |
|
|
|
Post by Bob on Jan 16, 2022 15:25:39 GMT -5
|
|
|
|
Post by Bob on Jan 16, 2022 15:28:01 GMT -5
Redo in tin ox has started. Those are rocks going in. All from 1,000 SC. I neglected to mention that I was knocking off the shine that way with all of them.
|
|
|
|
Post by Bob on Jan 23, 2022 18:33:16 GMT -5
Redo tin ox week 1 done. Just like before, on many rocks edges are super shiny but flats are dull. Does anyone know why this happens with tin ox? I tried to photo it on the glass. There is a distinct line between shiny and not.   |
|
|
|
Post by Mel on Feb 1, 2022 15:45:49 GMT -5
I'm not sure about the flat/dull debate....do you use any media in there? Maybe just one of those freakish things that happens because the rocks all fit together nicely?
They look very nice anyways!
|
|