|
|
Post by 1dave on Oct 17, 2020 11:35:18 GMT -5
Traces of million-year-old supernova found in Earth's deep sea www.inverse.com/science/ancient-supernova-in-the-deep-seaAs a star nears the end of its life, it runs out of fuel and collapses under the weight of its own gravity. The tremendous disintegration of the giant stellar being happens so quickly that it results in an extremely bright, powerful explosion known as a supernova.  Supernovae occur throughout the universe, and the material ejected by a star's explosive death spreads across the cosmos in a fiery flame. More like this Science 10.16.2020 7:04 AM The Moon may have acted as a protective shield for a young Earth By Passant Rabie Science 10.15.2020 8:00 AM Star cluster discovery stumps astronomers By Passant Rabie Science 10.14.2020 1:50 PM Scientists measured a key ingredient in star formation for the first time By Passant Rabie EARN REWARDS & LEARN SOMETHING NEW EVERY DAY. Now, a team of astronomers from the Australian National University have discovered traces of an ancient supernova here on Earth, buried deep underwater in the Indian Ocean after traveling to Earth some 33,000 years ago. The discovery is detailed in a study published Monday in the Proceedings of the National Academy of Sciences. As a star explodes in a fiery death, material from the supernova erupts into space.NASA/JPL-Caltech/STScI/CXC/SAO Stars are the main source of the different chemical elements found in the universe. Almost all of the elements that we know of were at some point created during the life and death of a star. Stars burn by producing helium from the hydrogen found in their cores; they then create heavier elements later in their life cycle, through a process known as nuclear burning. When a star explodes, all of its material erupts into surrounding space. The cataclysm essentially shapes the interstellar medium, or the matter found in the space between different star systems in a given galaxy. Some of that material may travel through our own Solar System, and the Solar System itself often passes through clouds of such interstellar material. As it passes, some of that same material could make its way on to Earth, leaving an imprint on the planet's geological record. The researchers behind the new study found traces of radioactive iron isotopes in five sediment samples collected from the depths of the Indian Ocean. The iron isotope, known as 60Fe, is produced in stars and ejected into interstellar medium during a supernova explosion. This iron isotope is only found in cosmic rays and is not naturally produced on Earth. Their presence in the sediments indicate that they likely traveled to Earth following a supernova explosion, the astronomers say. The iron was deposited on Earth at a rate of 3.5 atoms per square centimeter per year over the past 33,000 years, according to the study. This slow rate of deposition over a long period of time indicates that the supernova spread the iron isotopes throughout the interstellar medium. The origin of the radioactive iron was likely a million-year-old supernova which spread dust particles that may still be floating through interstellar space today, the study argues. The Local Interstellar Cloud (LIC) is the likeliest source for the iron, the study suggests The LIC is an interstellar cloud in the Milky Way galaxy that stretches roughly 30 light years across, and our Solar System has been moving through the LIC for thousands of years now. In fact, it is still in its midst. The LIC would contain remnants from ancient supernovae, which would make their way to Earth as our Solar System moves through this giant interstellar bubble. Further measurements are needed in order to build a better timeline of when exactly these isotopes made their way to Earth, and if they were in fact deposited by the LIC, the astronomers say. |
|
|
|
Post by mohs on Oct 17, 2020 14:59:26 GMT -5
That’s totally mind boggling Dave ! I’ll try to come up with something insightful to add might have to wait a couple more eons to past to allow the L. I. C. grit to settle in me  |
|
|
|
Post by stephan on Oct 17, 2020 15:23:52 GMT -5
Interesting stuff. I just looked it up, and iron has 4 stable isotopes ( 54Fe, 56Fe, 57Fe and 58fe) that occur on earth in varying abundance. Not too common to have that many stable isotopes. 60Fe abundance is listed as “trace,” and has a pretty substantial half-life. The other four add up to 100%, with the lowest being 0.28%! Even more interesting 55Fe and 59Fe only exist as synthetic materials. And detecting about 116,000 atoms per cm 2 is some impressive finding, given that there would be something on the order of 10 13-10 14 atoms in a monolayer of soil/dirt. That is ppl. If we think of the 116,000 of being in a cubic centimeter, the odds are even worse — about one in 10 17. 🤯 That’s WAY less likely than finding a Fairburn in jasoninsd ’s 21 ton landscaping rock pile.  Something to ponder |
|
|
|
Post by jasoninsd on Oct 17, 2020 17:40:28 GMT -5
Interesting stuff. I just looked it up, and iron has 4 stable isotopes ( 54Fe, 56Fe, 57Fe and 58fe) that occur on earth in varying abundance. Not too common to have that many stable isotopes. 60Fe abundance is listed as “trace,” and has a pretty substantial half-life. The other four add up to 100%, with the lowest being 0.28%! Even more interesting 55Fe and 59Fe only exist as synthetic materials. And detecting about 116,000 atoms per cm 2 is some impressive finding, given that there would be something on the order of 10 13-10 14 atoms in a monolayer of soil/dirt. That is ppl. If we think of the 116,000 of being in a cubic centimeter, the odds are even worse — about one in 10 17. 🤯 That’s WAY less likely than finding a Fairburn in jasoninsd ’s 21 ton landscaping rock pile.  Something to ponder Wait a second...how am I getting dragged into an Interstellar Quantum Physics discussion???  Are you saying the chances of me finding my Fairburns in my river rock were greater than me winning the Powerball lottery? I would've preferred to win the lottery...just sayin'!  |
|
|
|
Post by stephan on Oct 17, 2020 20:41:50 GMT -5
Interesting stuff. I just looked it up, and iron has 4 stable isotopes ( 54Fe, 56Fe, 57Fe and 58fe) that occur on earth in varying abundance. Not too common to have that many stable isotopes. 60Fe abundance is listed as “trace,” and has a pretty substantial half-life. The other four add up to 100%, with the lowest being 0.28%! Even more interesting 55Fe and 59Fe only exist as synthetic materials. And detecting about 116,000 atoms per cm 2 is some impressive finding, given that there would be something on the order of 10 13-10 14 atoms in a monolayer of soil/dirt. That is ppl. If we think of the 116,000 of being in a cubic centimeter, the odds are even worse — about one in 10 17. 🤯 That’s WAY less likely than finding a Fairburn in jasoninsd ’s 21 ton landscaping rock pile.  Something to ponder Wait a second...how am I getting dragged into an Interstellar Quantum Physics discussion???  Are you saying the chances of me finding my Fairburns in my river rock were greater than me winning the Powerball lottery? I would've preferred to win the lottery...just sayin'!  My brain makes unusual connections.... Afraid so. I remember having a homework assignment to calculate the odds of winning the lottery. Not good. Years later, I Remember laughing at eTrade’s slogan: ”Some is going to win the Lottery. Just not you.” Yup. |
|
|
|
Post by jasoninsd on Oct 17, 2020 21:40:00 GMT -5
Wait a second...how am I getting dragged into an Interstellar Quantum Physics discussion???  Are you saying the chances of me finding my Fairburns in my river rock were greater than me winning the Powerball lottery? I would've preferred to win the lottery...just sayin'!  My brain makes unusual connections.... Afraid so. I remember having a homework assignment to calculate the odds of winning the lottery. Not good. Years later, I Remember laughing at eTrade’s slogan: ”Some is going to win the Lottery. Just not you.” Yup. I have such admiration for [people] minds which have a true understanding of advanced mathematics. They are some of the few humans who have an opportunity to understand what perfection really is, as mathematics IS perfection...while those of us who have a basic, or even moderate understanding of mathematics only get a glimpse...but every once in a while, all a person may need is a glimpse to understand. I used to pray for a higher level of intelligence...now I just pray for wisdom. I've come to realize I've hit the lottery so many times during my existence...just not the Powerball.  |
|
|
|
Post by jasoninsd on Oct 17, 2020 21:52:09 GMT -5
1dave - If they're calculating the rate of deposit over a 33,000 year time period, yet the Earth has been traveling through the LIC (the likeliest source for the Iron) for thousands of years, I would imagine the rate of the iron deposits is greater now. Of course 2,000 and 33,000 can both be defined as "thousands", so without really knowing how long the earth has been traveling through the LIC, the rate could either be constant or far more variable. At least that's what I'm getting. Amazing science by the way...
|
|
|
|
Post by stephan on Oct 17, 2020 22:10:31 GMT -5
1dave - If they're calculating the rate of deposit over a 33,000 year time period, yet the Earth has been traveling through the LIC (the likeliest source for the Iron) for thousands of years, I would imagine the rate of the iron deposits is greater now. Of course 2,000 and 33,000 can both be defined as "thousands", so without really knowing how long the earth has been traveling through the LIC, the rate could either be constant or far more variable. At least that's what I'm getting. Amazing science by the way... I imagine it’s an average rate with significant variation. But with 60Fe having a half-life of 2,600,000 years, those variations would be difficult to detect. |
|
|
|
Post by jasoninsd on Oct 17, 2020 22:16:00 GMT -5
1dave - If they're calculating the rate of deposit over a 33,000 year time period, yet the Earth has been traveling through the LIC (the likeliest source for the Iron) for thousands of years, I would imagine the rate of the iron deposits is greater now. Of course 2,000 and 33,000 can both be defined as "thousands", so without really knowing how long the earth has been traveling through the LIC, the rate could either be constant or far more variable. At least that's what I'm getting. Amazing science by the way... I imagine it’s an average rate with significant variation. But with 60Fe having a half-life of 2,600,000 years, those variations would be difficult to detect. Dang. Every once in awhile my wife says she's low on iron for the month. If it was a constant, I could tell her she's getting bombarded with the same amount she was last month! But, since it's so variable, I better keep my mouth shut.  (Oh wait...not the same iron...)  |
|
|
|
Post by 1dave on Oct 18, 2020 7:08:59 GMT -5
1dave - If they're calculating the rate of deposit over a 33,000 year time period, yet the Earth has been traveling through the LIC (the likeliest source for the Iron) for thousands of years, I would imagine the rate of the iron deposits is greater now. Of course 2,000 and 33,000 can both be defined as "thousands", so without really knowing how long the earth has been traveling through the LIC, the rate could either be constant or far more variable. At least that's what I'm getting. Amazing science by the way... Earth is ALWAYS traveling through Supernova Debris! This STOMPED into my awareness when I began studying the Upheaval Impact 25 miles east of Moab Utah! That asteroid was composed of two parts vanadium, one part uranium, + tantalum, niobium, chromium, and they haven't studied what else.
LOOK AT THAT BATCH OF ELEMENTS ON THE PERIODIC CHART OF THE ELEMENTS AND ASK YOURSELF - HOW DID THEY GET TOGETHER IN ONE SPOT?
Comets are not "leftover pieces of the formation of the solar system." Think about planets spinning around a star that goes supernova. The planets are ripped into huge chunks. along with the heavy elements created in the explosion have a lot of gravitational pull. Those pieces are also in the cloud, adding comets, asteroids and meteors to the mix. We are floating through mine fields! en.wikipedia.org/wiki/Cometary_knot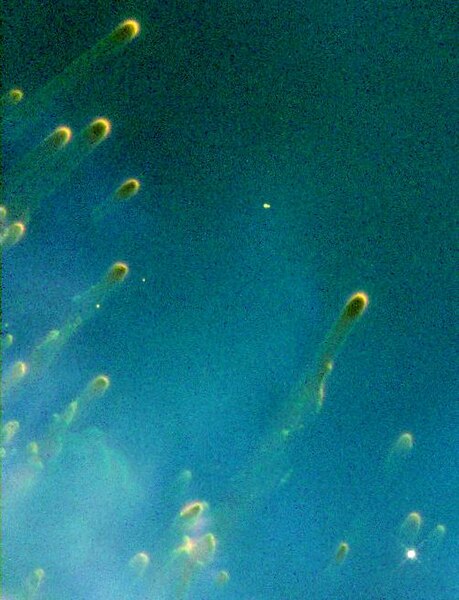 |
|
|
|
Post by mohs on Oct 18, 2020 9:09:41 GMT -5
What I’m getting from all this Is that the universe is a giant tumbler Were in the rough grit stage When will the rocks be ready for A.O. with a touch of sugar ? let it  |
|
|
|
Post by jasoninsd on Oct 18, 2020 9:16:19 GMT -5
What I’m getting from all this Is that the universe is a giant tumbler Were in the rough grit stage When will the rocks be ready for A.O. with a touch of sugar ? let it       I think you nailed it Mohs!!! |
|
|
|
Post by mohs on Oct 18, 2020 9:28:12 GMT -5
right on  jase here's  to a smooooth tumble RTH |
|
|
|
Post by mohs on Oct 18, 2020 12:17:58 GMT -5
Asteroid Bennu  take from Osiris-RE x That craft is going to attempt to vacuum up some grit from the asteroid and return to earth  jus because |
|
|
|
Post by jasoninsd on Oct 18, 2020 12:52:29 GMT -5
Asteroid Bennu  take from Osiris-RE x That craft is going to attempt to vacuum up some grit from the asteroid and return to earth  jus because Must be for you!  I was just reading on Bennu a couple weeks ago... |
|
|
|
Post by parfive on Oct 19, 2020 1:34:52 GMT -5
|
|
|
|
Post by parfive on Oct 21, 2020 17:25:26 GMT -5
|
|
|
|
Post by parfive on Oct 24, 2020 13:43:59 GMT -5
|
|













 jase
jase  to a smooooth tumble
to a smooooth tumble 












