Post by 1dave on Apr 1, 2022 9:05:47 GMT -5
When water mixes with hot, dry rock, they cause chemical bonds to break, so that the rock begins to melt at a lower temperature.
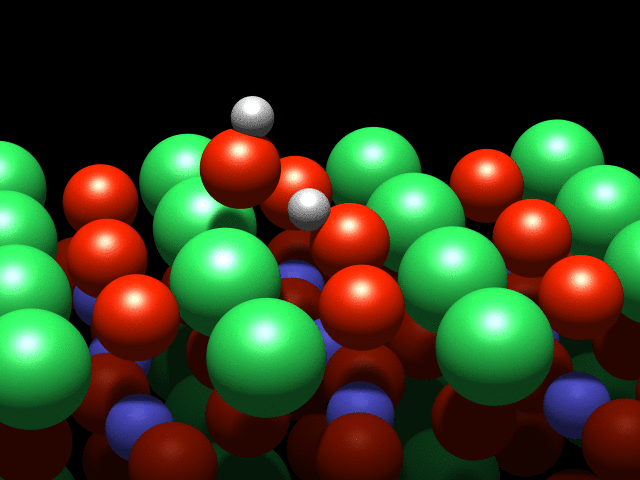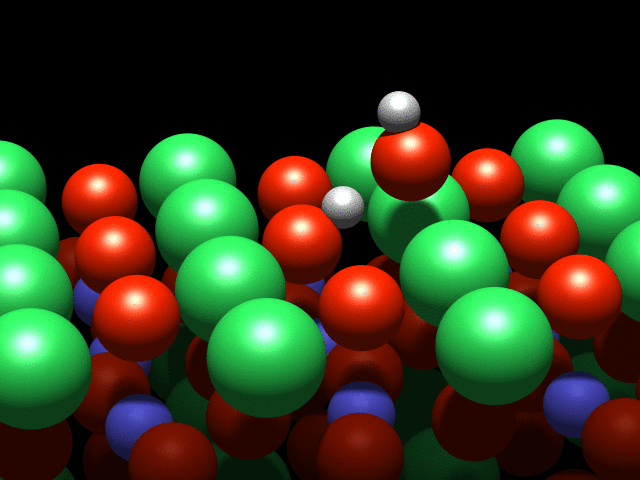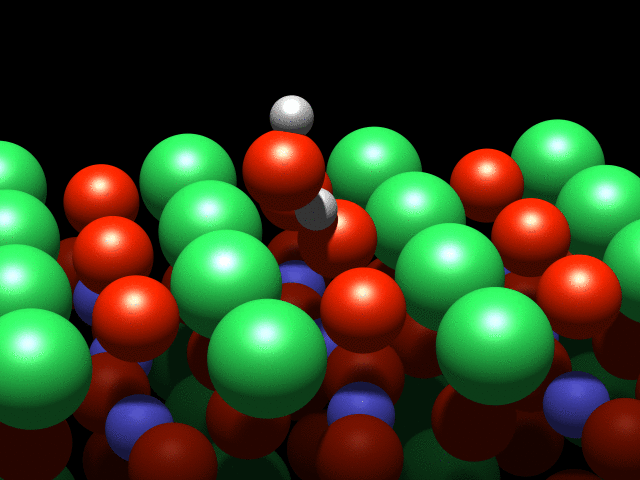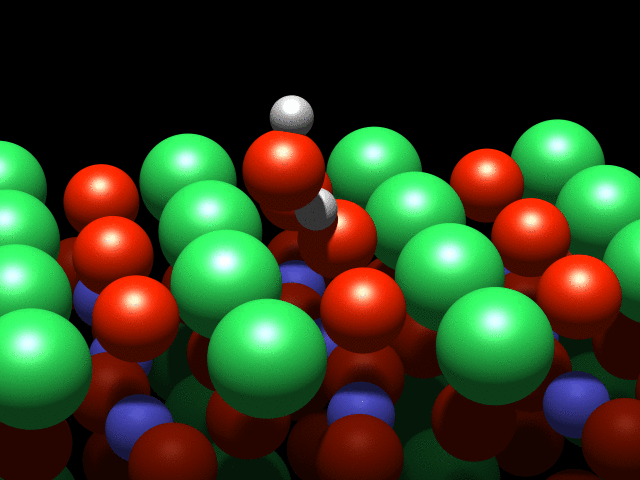
A visualization of the dance of the atoms on a crystal surface. Credit: TU Wien
Perovskites are materials used in batteries, fuel cells, and electronic components, and occur in nature as minerals. Despite their important role in technology, little is known about the reactivity of their surfaces. Professor Ulrike Diebold's team at TU Wien (Vienna) has answered a long-standing question using scanning tunnelling microscopes and computer simulations: How do water molecules behave when they attach to a perovskite surface? Normally only the outermost atoms at the surface influence this behaviour, but on perovskites the deeper layers are important, too.
The results have been published in the journal Nature Materials.
Perovskite dissociates water molecules
"We studied strontium ruthenate - a typical perovskite material," says Ulrike Diebold. It has a crystalline structure containing oxygen, strontium and ruthenium. When the crystal is broken apart, the outermost layer consists of only strontium and oxygen atoms; the ruthenium is located underneath, surrounded by oxygen atoms.
A water molecule that lands on this surface splits into two parts: A hydrogen atom is stripped off the molecule and attaches to an oxygen atom on the crystal's surface. This process is known as dissociation. However, although they are physically separated, the pieces continue to interact through a weak "hydrogen bond".
It is this interaction that causes a strange effect: The OH group cannot move freely, and circles the hydrogen atom like a dancer spinning on a pole. Although this is the first observation of such behaviour, it was not entirely unexpected: "This effect was predicted a few years ago based on theoretical calculations, and we have finally confirmed it with our experiments" said Diebold.
Dancing requires space
When more water is put on to the surface, the stage becomes too crowded and spinning stops. "The OH group can only move freely in a circle if none of the neighbouring spaces are occupied," explains Florian Mittendorfer, who performed the calculations together with PhD student Wernfried Mayr-Schmölzer. At first, when two water molecules are in neighbouring sites, the spinning OH groups collide and get stuck together, forming pairs. Then, as the amount of water is increased, the pairs stick together and form long chains. Eventually, water molecular cannot find the pair of sites it needs to split up, and attaches instead as a complete molecule.
Ulrike Diebold, Daniel Halwidl, Wernfried Mayr-Schmölzer, Florian Mittendorfer. Credit: TU Wien
The new methods that have been developed and applied by the TU Wien research team have made significant advances in surface research. Whereas researchers were previously reliant on indirect measurements, they can now - with the necessary expertise - directly map and observe the behaviour of individual atoms on the surface. This opens up new possibilities for modern materials research, for example for developing and improving catalysts.
Pole dancing water molecules: How water learns to dance

A visualization of the dance of the atoms on a crystal surface. Credit: TU Wien
Perovskites are materials used in batteries, fuel cells, and electronic components, and occur in nature as minerals. Despite their important role in technology, little is known about the reactivity of their surfaces. Professor Ulrike Diebold's team at TU Wien (Vienna) has answered a long-standing question using scanning tunnelling microscopes and computer simulations: How do water molecules behave when they attach to a perovskite surface? Normally only the outermost atoms at the surface influence this behaviour, but on perovskites the deeper layers are important, too.
The results have been published in the journal Nature Materials.
Perovskite dissociates water molecules
"We studied strontium ruthenate - a typical perovskite material," says Ulrike Diebold. It has a crystalline structure containing oxygen, strontium and ruthenium. When the crystal is broken apart, the outermost layer consists of only strontium and oxygen atoms; the ruthenium is located underneath, surrounded by oxygen atoms.
A water molecule that lands on this surface splits into two parts: A hydrogen atom is stripped off the molecule and attaches to an oxygen atom on the crystal's surface. This process is known as dissociation. However, although they are physically separated, the pieces continue to interact through a weak "hydrogen bond".
It is this interaction that causes a strange effect: The OH group cannot move freely, and circles the hydrogen atom like a dancer spinning on a pole. Although this is the first observation of such behaviour, it was not entirely unexpected: "This effect was predicted a few years ago based on theoretical calculations, and we have finally confirmed it with our experiments" said Diebold.
Dancing requires space
When more water is put on to the surface, the stage becomes too crowded and spinning stops. "The OH group can only move freely in a circle if none of the neighbouring spaces are occupied," explains Florian Mittendorfer, who performed the calculations together with PhD student Wernfried Mayr-Schmölzer. At first, when two water molecules are in neighbouring sites, the spinning OH groups collide and get stuck together, forming pairs. Then, as the amount of water is increased, the pairs stick together and form long chains. Eventually, water molecular cannot find the pair of sites it needs to split up, and attaches instead as a complete molecule.
Ulrike Diebold, Daniel Halwidl, Wernfried Mayr-Schmölzer, Florian Mittendorfer. Credit: TU Wien
The new methods that have been developed and applied by the TU Wien research team have made significant advances in surface research. Whereas researchers were previously reliant on indirect measurements, they can now - with the necessary expertise - directly map and observe the behaviour of individual atoms on the surface. This opens up new possibilities for modern materials research, for example for developing and improving catalysts.











