hypodactylus
spending too much on rocks
 
Member since July 2021
Posts: 477
|
Post by hypodactylus on Jul 14, 2022 0:16:25 GMT -5
So, I had read that some pretty common rocks can be a bit radioactive and decided to pick up a Geiger–Müller counter. While most of my rocks didn't seem to increase the CPM reading much (if at all), I came across some with elevated CPM counts. Information online seems to be a bit all over the place and perhaps more focused on generalized exposure. I am seeking some information from fellow rock collectors or sources of information on the topic. I like collecting rocks, but I don't understand radiation very well and it makes me a bit concerned/curious. The first rock that caught my attention was a piece of a chalcedony nodule from Utah; it was reading up to around 136CPM. (FYI, the background reading in that room of the house was around 25-30 CPM).
Video link (click the image):
The most 'active' rocks in my collection seem to generally be the agatized dino gem bone; many specimens were reading in the 50 - 100 CPM range, one in the 120 CPM range, and one in the 175 CPM range.  Background Reading: Brazilian Agate for reference:
Anyhow, just looking for any available commentary, wisdom, experience, and resources. Thank you!
|
|
|
|
Post by vegasjames on Jul 14, 2022 1:38:43 GMT -5
Even your background radiation is a bit high. Here is Las Vegas I get an average of 12-14 CPM for background radiation. The level considered problematic is around 50 CPM and up. Dino bone, some petrified wood and some opal and chalcedony can contain radioactive elements such as uranium. Normally the uranium will give a green glow under UV light, but not always. The carnotite (potassium vanadium uranium hydrate) I collect does not fluoresce under UV. For example, these chalcedonies covered with carnotite, bindheimite (lead antimony oxide hydroxide) and most likely oxyplumboroméite (lead antimony oxide) also found in the area, but looks just like the carnotite.  20211231_142834 20211231_142834 by James Sloane, on Flickr  20211231_142858 20211231_142858 by James Sloane, on Flickr Some pieces also contain opal such as this piece.  20211231_143109 20211231_143109 by James Sloane, on Flickr The carnotite portion does not fluoresce, although there are a couple other areas that do.  20220101_011854 20220101_011854 by James Sloane, on Flickr  20220101_011624 20220101_011624 by James Sloane, on Flickr Radiation levels are lower than I expected with the carnotite, which I assume is due to the lead from the bindheimite and oxyplumboroméite. Still, handling it for any length of time makes my hand tingle and I keep it stored outside for the moment. At the 81 and 175 CPM levels I would definitely minimize exposure. And keep in a sealed container if keeping it inside. Normally, uranium ores have a much higher CPM count than that, but still 50CPM is where they say precautions need to start being taken. |
|
|
|
Post by 1dave on Jul 14, 2022 7:32:45 GMT -5
So, I had read that some pretty common rocks can be a bit radioactive and decided to pick up a Geiger–Müller counter. While most of my rocks didn't seem to increase the CPM reading much (if at all), I came across some with elevated CPM counts. Information online seems to be a bit all over the place and perhaps more focused on generalized exposure. I am seeking some information from fellow rock collectors or sources of information on the topic. I like collecting rocks, but I don't understand radiation very well and it makes me a bit concerned/curious. The first rock that caught my attention was a piece of a chalcedony nodule from Utah; it was reading up to around 136CPM. (FYI, the background reading in that room of the house was around 25-30 CPM). The most 'active' rocks in my collection seem to generally be the agatized dino gem bone; many specimens were reading in the 50 - 100 CPM range, one in the 120 CPM range, and one in the 175 CPM range.  Background Reading: Brazilian Agate for reference:
Anyhow, just looking for any available commentary, wisdom, experience, and resources. Thank you!
The Real Midwest became uranium infested at the end of the Jurassic. Right on top of the Morrison Formation. 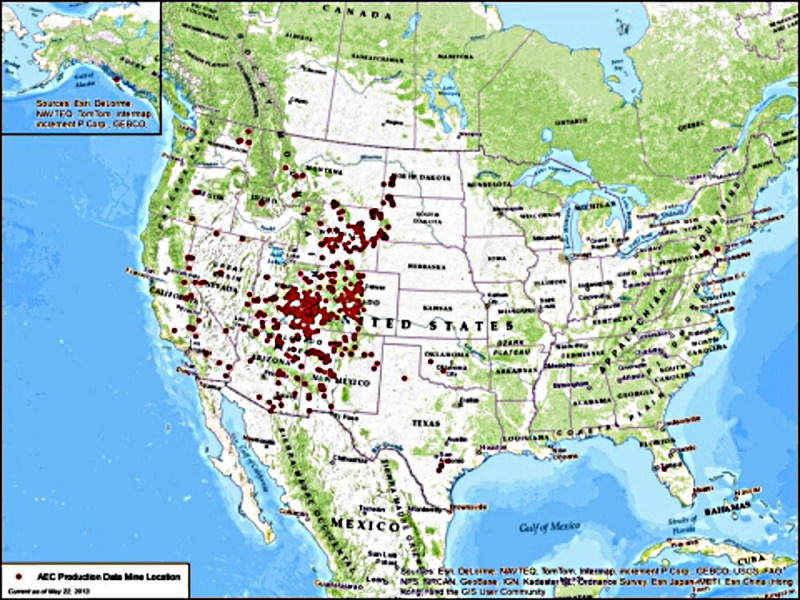 Uranium minerals are water soluble and make many rocks from theses areas fluorescent. |
|
hypodactylus
spending too much on rocks
 
Member since July 2021
Posts: 477
|
Post by hypodactylus on Jul 14, 2022 12:26:46 GMT -5
Even your background radiation is a bit high. Here is Las Vegas I get an average of 12-14 CPM for background radiation. Yeah, the 20 - 30 CPM background reading is pretty consistent at my house, inside and outside. I don't know if I am super concerned about storing the rocks, but I'll certainly have to think about it and learn more. Even positioning the counter four inches away from the rocks makes a huge difference in the reading; a reading of 140 CPM in one of the rocks goes down to 40 CPM at a four inch distance. |
|
hypodactylus
spending too much on rocks
 
Member since July 2021
Posts: 477
|
Post by hypodactylus on Jul 14, 2022 12:31:23 GMT -5
The Real Midwest became uranium infested at the end of the Jurassic. Right on top of the Morrison Formation. ... Uranium minerals are water soluble and make many rocks from theses areas fluorescent. Yeah, this is kind of why I was interested in getting a Geiger counter. I have acquired some rocks from a collection that was built largely from rocks found around the Morrison Formation in Utah. I had heard that petrified wood from this area can be radioactive. I haven't found any radioactive petrified wood in my collection (yet), but I did find the stuff mentioned above. |
|
|
|
Post by vegasjames on Jul 14, 2022 18:03:55 GMT -5
Even your background radiation is a bit high. Here is Las Vegas I get an average of 12-14 CPM for background radiation. Yeah, the 20 - 30 CPM background reading is pretty consistent at my house, inside and outside. I don't know if I am super concerned about storing the rocks, but I'll certainly have to think about it and learn more. Even positioning the counter four inches away from the rocks makes a huge difference in the reading; a reading of 140 CPM in one of the rocks goes down to 40 CPM at a four inch distance. Decay of uranium also produces the radioactive gas radon. Don't know how fast, but I would still display them sealed to be on the safe side. |
|























