|
|
Post by 1dave on Nov 18, 2013 10:42:07 GMT -5
Fabrication covers a lot of territory. It is a small part of Silversmithing. Here is a starting directory: Buying Gold & Silver1. Books.2. a Workbench3. Refining Silver4. Making Plate Why buy it when you can make it from your scrap? 5. Wire Art6. Wire wrapping 7. Precious Metal Clay8. Casting9. Tools10.Anvils11. Etching12. ElectroplatingAnnealingBlue FusePolishingEdinburgh EtchingTexturing MetalRepousseMokumeJump RingsRing ShanksHeatAdrian's CaneAdrian's Meteorite KnifeCab Pendant TutorialTutorial Turquoise and sapphires PendantEconomics-Studies-Demand-StylesConverting Lead into Gold!I've posted these images before, but this is all fabrication work: Many years ago I watched Don Black make jewelry for the tourists and rockhounds as they watched at the Spencer Precious Opal Mine shop in Spencer Idaho. I was amazed at how few (and large) tools he used to make masterpieces. About all he ever used was scrap sterling silver, a big pair of tin snips, a huge file, a hammer, chisel, a few punches, a ring mandrel, a chunk of wood, a torch, a burnisher, and a quick mind! He would take their stones, fit a bezel to it, place it on some sheet silver, hammer a few patterns into scrap silver he had just snipped from a previous project and place it around the bezel, remove the stone, solder everything together, perhaps solder a ring shank or whatever on the back, quench it in "pickle," polish it up, set the stone with the burnisher, and make the sale in about 5 minutes! Then on to the next customer. He was amazing! ADMIN EDIT - repaired broken images, original code below
[img alt="DavesJ" src="http://i1298.photobucket.com/albums/ag56/DaveCrosby/Metalsmithiing/2013-11-17-NovTools003b_zpsedba66dd.jpg" style="max-width:100%;"]
[img alt="Navajo-1" src="http://i1298.photobucket.com/albums/ag56/DaveCrosby/Metalsmithiing/2013-11-17-NovTools007m_zps6c8dd8a7.jpg" style="max-width:100%;"]
[img alt="Navajo-2" src="http://i1298.photobucket.com/albums/ag56/DaveCrosby/Metalsmithiing/2013-11-17-NovTools006m_zps3894a091.jpg" style="max-width:100%;"][/quote]
[img alt="SSTools" src="http://i1298.photobucket.com/albums/ag56/DaveCrosby/Metalsmithiing/01-SilverSmithTools-1_zpscb4838a4.jpg" style="max-width:100%;"][/quote]
|
|
|
|
Post by 1dave on Nov 18, 2013 10:44:26 GMT -5
A handy tool for bending and smoothing the bezel around your stone is a burnisher.  I found a 1/4" X 1 1/4 X 2" Chunk of iron stock rounded on one end and polished worked better for me. I can't find my old one, but found a blank from the last time I made a bunch for friends. Saw off the torched end and a little polishing and I may even do something again.  |
|
|
|
Post by 1dave on Nov 19, 2013 0:20:33 GMT -5
Faantastic Fabrication by jamesp: I have made a few railroad track anvils over the years for the local jewelry school. I sand and polish them w/wet diamond pads. Saw them with a cut off to not heat them and soften them. Purchase all the silver plate that i find cheap. The base metal in that old stuff is highest quality white brass, brass and copper. Hammers very well on the anvil. ALL people going into silver/mixed metal should practice on copper roofing/wire/silver plated trays. Soft stainless too. These are the edges of a silver plate tray. Floral is pewter. Base is brass. Coating is silver.  All of these were made out of copper wire and silver plate trays. Skip the grits and polish direct with 1 HP grinder and 8 inch buffer pads constantly coated w/fresh tripoli. They do get hot  virus  disease  a trachea  |
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,602
|
Post by jamesp on Nov 19, 2013 0:44:05 GMT -5
Thanks Dave. That was all tinkering and practice. I really like hammer forming. I have a large tin snip i put in a vice and cut sheet copper,brass,stainless into jewelry size pieces and then tumble them with granite pea gravel to round off the cuts. Cut up about 6-7 pounds and then run them with 9-10 pounds of the gravel. Sure saves on filing and burr removal.
|
|
gemfeller
Cave Dweller 
Member since June 2011
Posts: 4,059 
|
Post by gemfeller on Nov 19, 2013 21:08:06 GMT -5
James and Dave, you are people of many talents and enthusiasms. You've tempted me -- I'm close to clearing all the rocks off my jewelry bench and start some silver projects. I didn't make this bracelet; I acquired it years ago and dug it out today. I'm posting it because it's a nice example of silver fabrication and I'd like to get Dave's opinion on the variscite. I'm sure it's Clay Canyon material with inclusions of what??? Wavellite? Crandallite? Other?   Rick ADMIN EDIT - repaired broken images, original code: [a href="http://s1316.photobucket.com/user/gemfeller/media/DSCN0876_zps205b3cbe.jpg.html"]
[img src="http://i1316.photobucket.com/albums/t620/gemfeller/DSCN0876_zps205b3cbe.jpg" style="max-width:100%;"][/a]
[a href="http://s1316.photobucket.com/user/gemfeller/media/DSCN0888_zps9ab0e665.jpg.html"]
[img src="http://i1316.photobucket.com/albums/t620/gemfeller/DSCN0888_zps9ab0e665.jpg" style="max-width:100%;"][/a] |
|
|
|
Post by 1dave on Nov 20, 2013 11:36:15 GMT -5
James and Dave, you are people of many talents and enthusiasms. You've tempted me -- I'm close to clearing all the rocks off my jewelry bench and start some silver projects. I didn't make this bracelet; I acquired it years ago and dug it out today. I'm posting it because it's a nice example of silver fabrication and I'd like to get Dave's opinion on the variscite. I'm sure it's Clay Canyon material with inclusions of what??? Wavellite? Crandallite? Other? Rick Hi Rick, I'd agree with the "enthusiasms" part. Yesterday I decided to show talkingstones how to build that copper bracelet I described. I got some #6 solid copper wire and thought rather than electrical solder I would dig around and find the silver solder I bought back before I closed my rock shop in 1970. Found it! The 1X2" sheets of medium I had marked to sell at $3. 00 each. There were 5 small zip-lock bags of 1/4X11" strips marked "H-185" that I had never used before. "Hard solder, Perfect!" I thought. I cut a snippet of it and wedged it into the joint I wished to solder, doused everything with Sparex and turned my torch on to the copper on each side of the solder to bring it up to melting temperature. Nothing! When firescale started forming, I did a supposed no-no and put the tip of the flame directly on the square of solder. 5 minutes later the corners were just as square as when I started. No melting whatsoever! I don't know what that stuff is, but if I had been working on something valuable, it would have been ruined! Sterling would have been melted. Too bad I can't even remember who I bought it from, but it ain't hard silver solder! I am not where I was with all this stuff 40 years ago! Top craftsman work on the bracelet! Thanks for sharing!!! I agree that is Clay Canyon. I'm no expert on the altered forms, just what I read: |
|
|
|
Post by pghram on Nov 22, 2013 9:42:36 GMT -5
That's an outstanding piece, thanks for sharing.
Rich
|
|
Deleted
Deleted Member
Member since January 1970
Posts: 0
|
Post by Deleted on Nov 22, 2013 13:56:43 GMT -5
I'm sure it's Clay Canyon material with inclusions of what??? Wavellite? Crandallite? Other?   Rick Bauxite seems to grow like that and have similar colors. Why not aluminum oxide hydroxide in the aluminum phosphate?  |
|
gemfeller
Cave Dweller 
Member since June 2011
Posts: 4,059 
|
Post by gemfeller on Nov 22, 2013 15:12:03 GMT -5
Anything's possible, Scott. But the mineral associations in Clay Canyon variscite have been studied in depth. Telling one from another, however, is work for a qualified mineralogist, not me. Here's what Mindat has to say (in an article posted there by 1Dave):
American Mineralogist Volume 27, pages 441-451, 1942
THE MINERALOGY AND PARAGENESIS OF THE VARISCITE NODULES FROM NEAR FAIRFIELD, UTAH. PART 3.
ESPER S. LARSEN, 3d., Harvard University, Cambridge, Mass.
TABLE OF CONTENTS. PART 3.
Origin of the variscite and later phosphates . . . . . . . . . . . . . . . . . . . . . . . .441
Character of the depositing solutions . . . . . . . . . . . . . . . . . . . . . . . . . . . . 441
Source of the phosphate material . . . . . . . . . . . .. . . . . . . . .. . . . . . . .. . . 444
Geochemistry of phosphates . . . .. . . . . .. . . . . . . . . . . . . . .. . . . . . . . . . .445
Application of the geochemistry to the Fairfield deposit . . . . . . . . . . . . . . 446
Summary of origin . . .. .. . . . . . . . . .. . . . . . . . . . . . . . . .. . . .. .. . . . . . 449
References . . .. .. . . . . . . . . . . . . .. . . . . . . .. . . . . . . .. . . . . . . . . . . . 450
* * *
SUMMARY OF ORIGIN
Phosphorite beds at the surface were attacked by carbonated surface waters, and the resultant solutions of calcium phosphate moved downward along throughgoing channels into the zone below the water table. Here the solutions traversed aluminous material, perhaps shales, and deposited the phosphate as the aluminum salt, variscite. This reaction perhaps caused a decrease in the alkalinity of the solutions. When the phosphorite was completely removed at the surface, the downward-moving ground waters became free of phosphate material, and returned to their usual alkaline state.
This return to stronger alkalinity caused a reaction with the variscite to replace it with calcium aluminum phosphate (pseudowavellite), an introduction of only calcium. Soda then became an important constituent of the solutions, perhaps derived from the weathering of shales or shaly limestones exposed at the surface; this resulted in the deposition of millisite and wardite. The soda increased the alkalinity of the solutions, eventually to the point where the deposition of wardite did not keep pace with the removal of variscite by solution. The solutions then returned to their normal alkalinity, probably with the removal of the shale and the exposure again of limestones, and again deposited pseudowavellite in place of the variscite. This ended the bulk of the mineralization.
The stages following this are represented in the nodules primarily by rare crystals in the solution cavities, at first aluminum phosphates of magnesium (gordonite), followed by calcium. In the final stage aluminum is absent, and alkalis reappear. The equilibrium conditions controlling the deposition of these is not known, so that the state of the solutions forming them cannot be surmised. With the lowering of the water table below the variscite, oxidizing conditions ensued, with the deposition of abundant limonite.
It is believed that one of the major factors permitting the deposition of variscite at Fairfield, and probably some other deposits, was the existence of open fissures which permitted the surface solutions containing dissolved calcium phosphate to move rapidly downward through underlying limestone into rocks containing aluminum. In the normal course, ground waters with dissolved calcium phosphate seep down into underlying limestones, where the phosphate is leisurely precipitated by the excess of calcium carbonate.
Where open channels allow more rapid descent of the solutions, the precipitating effect of the limestone is not so effective, and phosphate-bearing solutions can thus reach aluminous rocks, and the aluminum will act as the precipitant. The equilibrium conditions which determined the deposition of variscite rather than some other aluminum phosphate (such as wavellite) are not known.
|
|
Deleted
Deleted Member
Member since January 1970
Posts: 0
|
Post by Deleted on Nov 22, 2013 15:57:33 GMT -5
with all that going on I see no reason for aluminum phosphate to form and then decompose to bauxite.
|
|
|
|
Post by 1dave on Jan 31, 2014 13:38:46 GMT -5
I edited the first post to add a directory at the front.
|
|
|
|
Post by 1dave on Jan 14, 2017 13:03:07 GMT -5
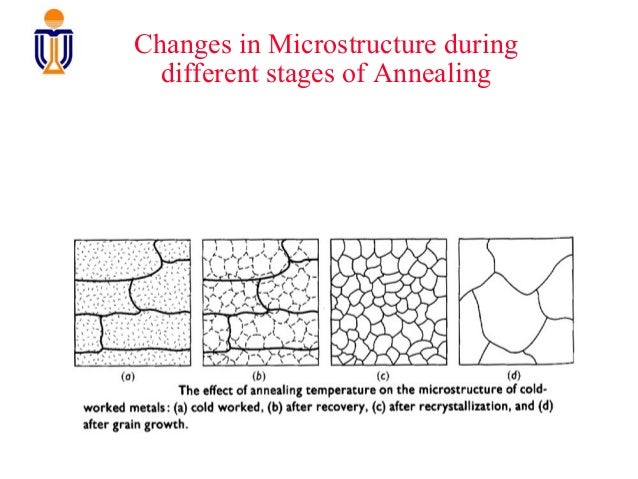
Annealing  Annealing newly drawn silver wire.  Reloaders annealing Work Station |
|
|
|
Post by pauls on Mar 7, 2017 17:26:56 GMT -5
A handy tool for burnishing a bezel is a piece of tumbled Agate, you can actually buy one with a handle but I just grab whatever handy sized chunk of tumbled Agate I have close.
If you want to make a good burnishing tool from metal the shank from a broken drill bit is tool steel, it can be ground down to shape and polished up on your rock sanding wheels then mounted in a file handle.
|
|
|
|
Post by Pat on Mar 7, 2017 17:33:38 GMT -5
pauls have you found the agate a better burnisher than the typical metal burnishers? It is a pretty tool!
|
|
|
|
Post by rockpickerforever on Mar 7, 2017 18:30:13 GMT -5
Google "agate burnishing tool". Many available, not very expensive. Is it worth it? IDK, never used one. I'm more into metal myself.
|
|
|
|
Post by 1dave on Mar 13, 2017 7:54:02 GMT -5
A handy tool for bending and smoothing the bezel around your stone is a burnisher.  I found a 1/4" X 1 1/4 X 2" Chunk of iron stock rounded on one end and polished worked better for me. I can't find my old one, but found a blank from the last time I made a bunch for friends. Saw off the torched end and a little polishing and I may even do something again.  |
|